Conversion of carboxylic acids to alcohols using LiAlH4
( \newcommand{\kernel}{\mathrm{null}\,}\)
Carboxylic acids can be converted to 1o alcohols using Lithium aluminum hydride (LiAlH4). Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.
General reaction
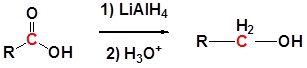
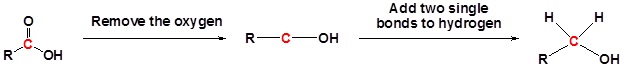
Example
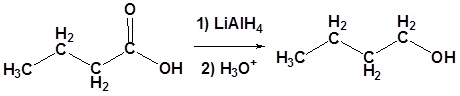
Possible Mechanism
1) Deprotonation
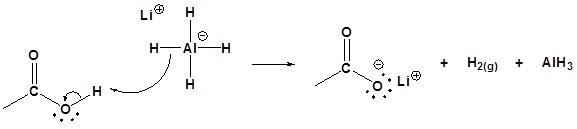
2) Nucleophilic attack by the hydride anion
3) Leaving group removal
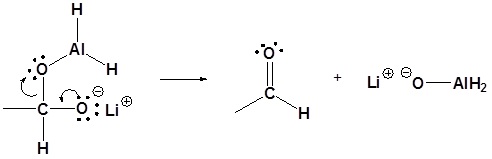
4) Nucleophilic attack by the hydride anion
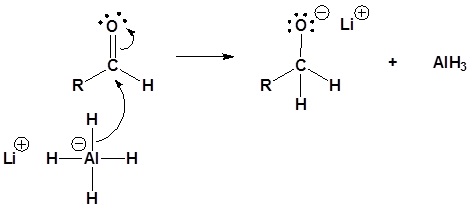
5) The alkoxide is protonated
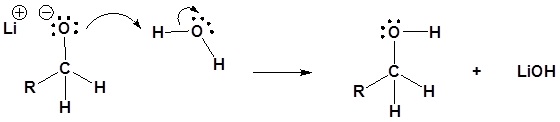
Contributors
Prof. Steven Farmer (Sonoma State University)

