Viscosity
- Page ID
- 1512
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Viscosity is another type of bulk property defined as a liquid’s resistance to flow. When the intermolecular forces of attraction are strong within a liquid, there is a larger viscosity. An example of this phenomenon is imagining a race between two liquids down a windshield. Which would you expect to roll down the windshield faster honey or water? Obviously from experience one would expect water to easily speed right past the honey, a fact that reveals honey has a much higher viscocity than water.
Introduction
Viscosity can be not only a fluid’s resistance to flow but also a gas’ resistance to flow, change shape or movement. The opposite of viscosity is fluidity which measures the ease of flow while liquids such as motor oil or honey which are “sluggish” and high in viscosity are known as viscous. One may ask the question of what is actually going on in the liquids to make one type flow faster and the other more resistant to flow such as the comparison between honey and water earlier. Because part of a fluid moves, it forces other adjacent parts of the liquid to move along with it causing an internal friction between the molecules which ultimately leads to a reduced rate of flow.
It is also important to note that the viscosity of liquids and gases are affected by temperature but in opposite ways meaning that upon heating, the viscosity of a liquid decreases rapidly, whereas gases flow more sluggishly. Why is this the case? As temperature increases, the average speed of molecules in a liquid also increases and as a result, they spend less time with their "neighbors." Therefore, as temperature increases, the average intermolecular forces decrease and the molecules are able to interact without being "weighed down" by one another. The viscosity of a gas, however, increases as temperature increases because there is an increase in frequency of intermolecular collisions at higher temperatures. Since the molecules are flying around in the void most of the time, any increase in the contact they have with one another will increase the intermolecular force which will ultimately lead to a disability for the whole substance to move.
Measuring Viscosity
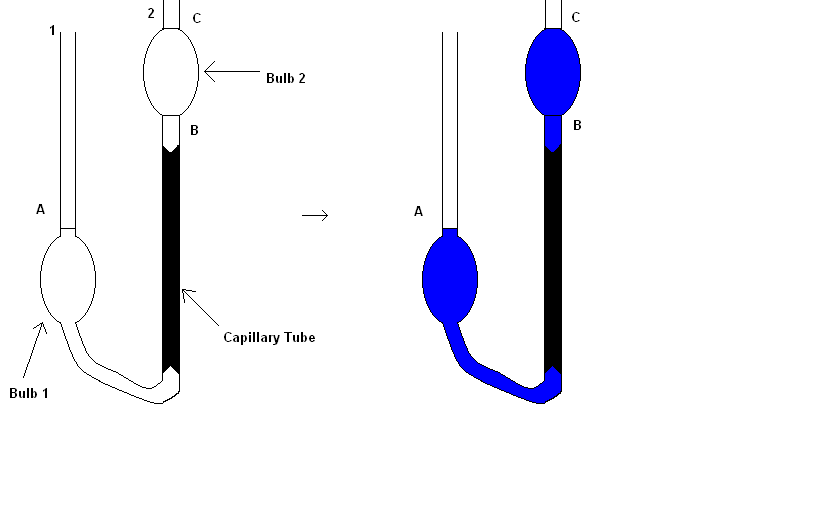
There are numerous ways to measure viscosity. One of the most elementary ways is to allow a sphere, such as a metal ball, to drop through a fluid and time the fall of the metal ball: the slower the sphere falls, the greater the viscosity that is measured. Another more advanced design of measuring viscosity known as the Ostwald Viscometer that is much more accurate than dropping a metal ball. An Ostwald Viscometer consists of two reservoir bulbs and a capillary tube. The viscometer is filled with liquid until the liquid reaches the mark A with the aid of a pipette to accurately measure out the volume of needed liquid. The viscometer is then put into a water bath which equilibrates the temperature of the test liquid. As noted before, the equilibration is important to maintain a constant temperature as to not affect the viscosity otherwise. The liquid is then drawn through the side 2 of the U-tube by use of suction and lastly, the flow is time between marks C and B. The viscosity is calculated with Equation \(\ref{1}\)
\[ \eta =K t \label{1}\]
where \(K\) is the value of a liquid with known viscosity and density such as water. Once the value of K is known, the viscosity can be determined by measuring the amount of time the test liquid flows between the two graduated marks.
Units of Measure:
1 Pascal-second (Pa•s) = 1 kg•m?1•s?1 1 Poise = 1 g•cm?1•s?1
Relationship between pascal-second to poise:
10 P = 1 kg•m?1•s?1 = 1 Pa•s 1 cP = 0.001 Pa•s = 1 mPa•s
When measuring viscosity with any type of viscometer, accurate temperature is so important that viscosity can double with a change of only 5 Celsius.
| Temperature | Viscosity |
|---|---|
| 290.00 | 96.102 |
| 295.00 | 75.392 |
| 300.00 | 59.906 |
| 305.00 | 48.167 |
| 310.00 | 39.153 |
| 315.00 | 32.150 |
| 320.00 | 26.649 |
| 325.00 | 22.283 |
| 330.00 | 18.785 |
| 335.00 | 15.956 |
| 340.00 | 13.651 |
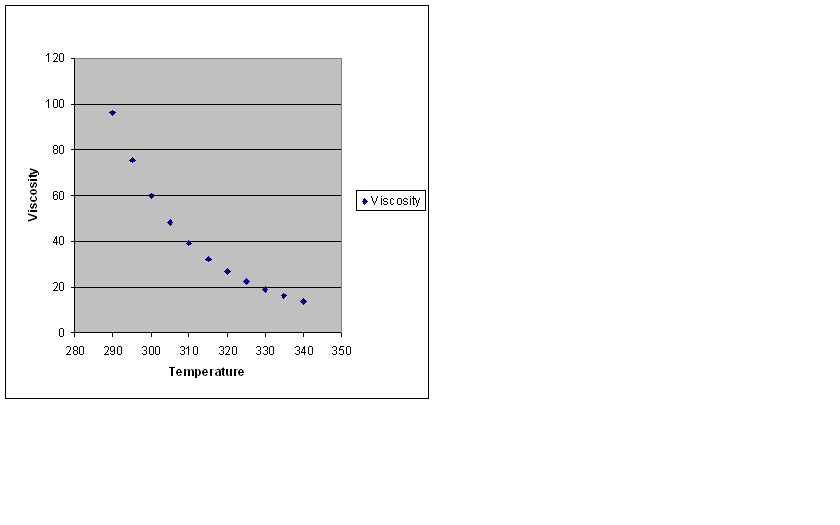
notice how the increase in temperature lowers the viscosity of the fluid which in this case is olive oil
- Pitch (any highly viscous liquid which appear solid) has a viscosity of nearly 100 billion times the viscosity of water
References
- Petrucci, et al. General Chemistry: Principles & Modern Applications. 9th ed. Upper Saddle River, New Jersey: Pearson/Prentice Hall, 2007.
- Viswanath, Dabir and Ghosh, Tushar and Prasad, Dasika and Dutt, Nidamarty, and Rani, Kalipatnapu. Viscosity of Liquids: Theory, Estimation, Experiment, and Data. Dordrecht, The Netherlands: Springer, 2007.
- Natarajan, G, and Viswanath, D. Data Book On The Viscosity of Liquids. United Sates of America: Hemisphere Publishing Corporation, 1989.
Contributors and Attributions
- Christopher Wen

