Reactions of Phenylamine as a Primary Amine
- Page ID
- 3994
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page looks at reactions of phenylamine (also known as aniline or aminobenzene) where it behaves as a fairly straightforward primary amine. It explains why phenylamine is a weaker base than other primary amines, and summarises its reactions with acyl chlorides (acid chlorides), acid anhydrides and halogenoalkanes (haloalkanes or alkyl halides). Before you read each section on this page, you should follow the link to the corresponding page about aliphatic amines (those not based on benzene rings). In most cases, the reactions are the same, and this page only really looks in detail at the differences in the phenylamine case.
Amines are bases because the lone pair of electrons on the nitrogen atom can accept a hydrogen ion - in other words, for exactly the same reason that ammonia is a base. With phenylamine, the only difference is that it is a much weaker base than ammonia or an amine like ethylamine - for reasons that we will explore later.
Reaction of Phenylamine with Acids
Phenylamine reacts with acids like hydrochloric acid in exactly the same way as any other amine. Despite the fact that the phenylamine is only a very weak base, with a strong acid like hydrochloric acid the reaction is completely straightforward. Phenylamine is only very slightly soluble in water, but dissolves freely in dilute hydrochloric acid. A solution of a salt is formed - phenylammonium chloride.
To show the formation of the salt, you could write:
\[ C_6H_5NH_2 + HCl \rightarrow C_6H_5NH_3^+ Cl^-\]
. . . or if you want to emphasize the fact that the phenylamine is acting as a base, you could most simply use:
\[ C_6H_5NH_2 (l) + H^+ (aq) \rightarrow C_6H_5NH_3^+\]
To get the phenylamine back from the phenylammonium ion present in the salt, all you have to do is to take the hydrogen ion away again. You can do that by adding any stronger base. Normally, you would choose sodium hydroxide solution:
\[ C_6H_5NH_3^+ (aq) + OH^- (aq) \rightarrow C_6H_5NH_2 + H_2O (l) \]
The phenylamine is formed first as an off-white emulsion - tiny droplets of phenylamine scattered throughout the water. This then settles out to give an oily bottom layer of phenylamine under the aqueous layer.
Reactions of Phenylamine with Ethers
This is where it is possible to tell that phenylamine is a much weaker base than ammonia and the aliphatic amines like methylamine and ethylamine. Phenylamine reacts reversibly with water to give phenylammonium ions and hydroxide ions.
\[ C_6H_5NH_2 (aq) + H_2O (l) \rightleftharpoons C_6H_5NH_3^+ + OH^- (aq)\]
The position of equilibrium lies well to the left of the corresponding ammonia or aliphatic amine equilibria - which means that not many hydroxide ions are formed in the solution. The effect of this is that the pH of a solution of phenylamine will be quite a bit lower than a solution of ammonia or one of the aliphatic amines of the same concentration. For example, a 0.1 M phenylamine solution has a pH of about 9 compared to a pH of about 11 for 0.1 M ammonia solution.
Amines are bases because they pick up hydrogen ions on the lone pair on the nitrogen atom. In phenylamine, the attractiveness of the lone pair is lessened because of the way it interacts with the ring electrons. The lone pair on the nitrogen touches the delocalized ring electrons . . .
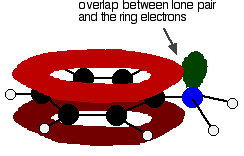
. . . and becomes delocalized with them:
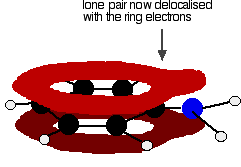
That means that the lone pair is no longer fully available to combine with hydrogen ions. The nitrogen is still the most electronegative atom in the molecule, and so the delocalized electrons will be attracted towards it, but the electron density around the nitrogen is nothing like it is in, say, an ammonia molecule. The other problem is that if the lone pair is used to join to a hydrogen ion, it is no longer available to contribute to the delocalization. That means that the delocalization would have to be disrupted if the phenylamine acts as a base. Delocalization makes molecules more stable, and so disrupting the delocalization costs energy and won't happen easily. Taken together - the lack of intense charge around the nitrogen, and the need to break some delocalization - means that phenylamine is a very weak base indeed.
Reactions of Phenylamine with Acyl Chlorides and with Acid Anhydrides
These are reactions in which the phenylamine acts as a nucleophile. There is no essential difference between these reactions and the same reactions involving any other primary amine. You will find a summary of the reactions below, but all the detailed explanations are on other pages. We'll take ethanoyl chloride as a typical acyl chloride, and ethanoic anhydride as a typical acid anhydride. The important product of the reaction of phenylamine with either of these is the same. Phenylamine reacts vigorously in the cold with ethanoyl chloride to give a mixture of solid products - ideally white, but usually stained brownish. A mixture of N-phenylethanamide (old name: acetanilide) and phenylammonium chloride is formed.
The overall equation for the reaction is:
\[ CH_3COCl + 2C_6H_5NH_2 \rightarrow CH_3CONHC_6H_5 + C_6H_5NH_3^+ Cl^-\]
With ethanoic anhydride, heat is needed. In this case, the products are a mixture of N-phenylethanamide and phenylammonium ethanoate.
\[ (CH_3CO)_2O + 2C_6H_5NH_2 \rightarrow CH_3CONHC_6H_5 + CH_3COO^-NH_3C_6H_5^+\]
The main product molecule (the N-phenylethanamide) is often drawn looking like this:
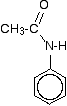
If you stop and think about it, this is obviously the same molecule as in the equation above, but it stresses the phenylamine part of it much more. Looking at it this way, notice that one of the hydrogens of the -NH2 group has been replaced by an acyl group - an alkyl group attached to a carbon-oxygen double bond. Tthe phenylamine has been acylated or has undergone acylation. Because of the nature of this particular acyl group, it is also described as ethanoylation. The hydrogen is being replaced by an ethanoyl group, CH3CO-.
Reactions of Phenylamine with Halogenoalkanes
This is another reaction of phenylamine as a nucleophile, and again there is no essential difference between its reactions and those of aliphatic amines. Taking bromoethane as a typical halogenoalkane, the reaction with phenylamine happens in the same series of complicated steps as with any other amine. We'll just look at the first step. On heating, the bromoethane and phenylamine react to give a mixture of a salt of a secondary amine and some free secondary amine. In this case, you would first get N-ethylphenylammonium bromide:
![]()
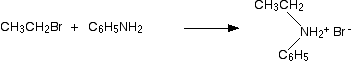
This would instantly be followed by a reversible reaction in which some unreacted phenylamine would take a hydrogen ion from the salt to give some free secondary amine: N-ethylphenylamine.
![]()
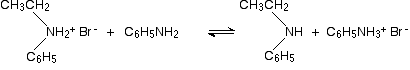
The reaction would not stop there. You will get further reactions to produce a tertiary amine and its salt, and eventually a quaternary ammonium compound. If you want to explore this further, refer to the last link just up the page, and trace the sequence of equations through using phenylamine rather than ethylamine.
Contributors
Jim Clark (Chemguide.co.uk)


