Physical Properties of Ether
- Page ID
- 35774
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Dimethylether and ethyl methyl ether are gases at ordinary temperature. The other lower homologues are colorless, pleasant smelling, volatile liquids with typical ether smell.
Boiling points
The C - O bonds in ether are polar and thus ethers have a net dipole moment. The weak polarity of ethers do not appreciably affect their boiling points which are comparable to those of the alkenes of comparable molecular mass. Ethers have much lower boiling points as compared to isomeric alcohols. This is because alcohols molecules are associated by hydrogen bonds while ether molecules are not.
Solubility
Ethers containing up to 3 carbon atoms are soluble in water, due to their hydrogen bond formation with water molecules.
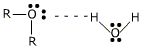
The solubility decreases with increase in the number of carbon atoms. The relative increase in the hydrocarbon portion of the molecule decreases the tendency of H-bond formation. Ethers are appreciably soluble in organic solvents like alcohol, benzene, acetone etc.
Structure of Ethers
Ethers are a class of organic compounds that contain an sp3 hybridized oxygen between two alkyl groups and have the formula R-O-R'. these compounds are used in dyes, perfumes, oils, waxes and other industrial uses. Aliphatic ethers have no aryl groups directly attached to the ether oxygen.
Examples of Aliphatic Ethers
Aromatic ethers have at least one aryl ring directly attached to the ether oxygen. In aryl ethers, the lone pair elections on oxygen are conjugated with the aromatic ring which significantly changes the properties of the ether.
Example of Aromatic Ethers
The sp3 hybridization of oxygen gives ethers roughly the same geometry as alcohols and water. The R-O-R' bond angle is close to what is expected in a tetrahedral geometry. The bond angle of dimethyl ether is 112o which is larger than the H-O-H bond angle in water (104.5o) due to the steric repulsion of the methyl groups.
The presence of an electronegative oxygen atom gives ethers a small dipole moment.
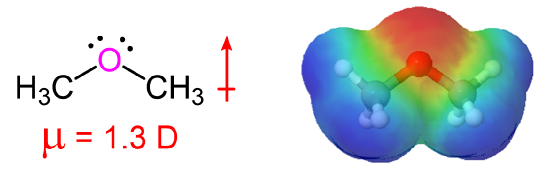
Comparisons of Physical Properties of Alcohols and Ethers
Ethers, unlike alcohols, have no hydrogen atom on the oxygen atom (that is, no OH group). Therefore, there is no intermolecular hydrogen bonding between ether molecules, which makes their boiling points much lower than an alcohol with similar mass. Despite the presence of a small dipole moment, ethers have boiling points that about the same alkanes of comparable molar mass. (Table 18.1.2).
| Condensed Structural Formula | Name | Molar Mass | Boiling Point (°C) | Intermolecular Hydrogen Bonding in Pure Liquid? |
|---|---|---|---|---|
| CH3CH2CH3 | propane | 44 | –42 | no |
| CH3OCH3 | dimethyl ether | 46 | –25 | no |
| CH3CH2OH | ethyl alcohol | 46 | 78 | yes |
| CH3CH2CH2CH2CH3 | pentane | 72 | 36 | no |
| CH3CH2OCH2CH3 | diethyl ether | 74 | 35 | no |
| CH3CH2CH2CH2OH | butyl alcohol | 74 | 117 | yes |
Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it. For example, dimethyl ether and ethanol (both having the molecular formula C2H6O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C4H10O) are barely soluble in water (8 g/100 mL of water).
Peroxide Formation
Many ethers can react with oxygen to form explosive peroxide compounds n a free radical process called autoxidation. For this reason ethers should not be stored for long periods of time and should not be stored in glass bottles. The danger is particularly acute when ether solutions are distilled to near dryness. The hydroperoxides can become more concentrated during a distillation because they tend to have a slightly higher boiling point than the corresponding ether. Before performing an ether distillation great care should be taken to test for the presence of peroxides.
Contributors
Binod Shrestha (University of Lorraine)

