Spectroscopy of the Alkynes
- Page ID
- 911
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Unlike alkenyl hydrogens, alkynyl hydrogens give rise to shielded hydrogens, or relatively high field chemical shifts for H-NMR when subjected to an external magnetic field. This can be explained by the cylindrical \(pi\) cloud around the carbon-carbon triple bond. Long range coupling is also observed in the alkynes. Infrared spectroscopy is a useful complement to NMR data, and displays characteristic peaks for terminal and internal alkynes.
NMR Absorptions of Alkyne Hydrogens
As discussed before, a carbon-carbon triple bond is the functional characteristic of the alkynes, and protons, or hydrogens, bound to these sp-hybridized carbon atoms resonate at ? = 1.7-3.1 ppm. For example, in the NMR spectrum of 3,3-dimethyl-1-butyne, the terminal hydrogen of the alkyne appears at ? = 2.06 ppm.
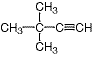 3,3-dimethyl-1-butyne.
3,3-dimethyl-1-butyne.
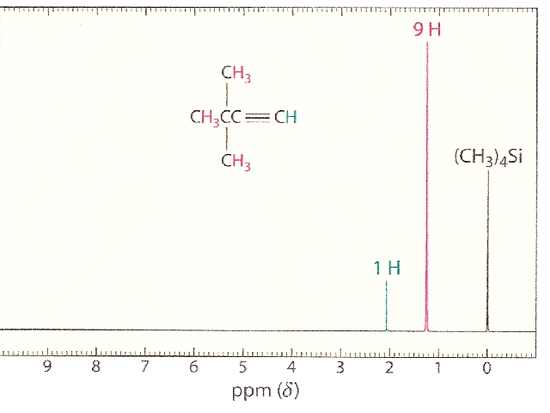
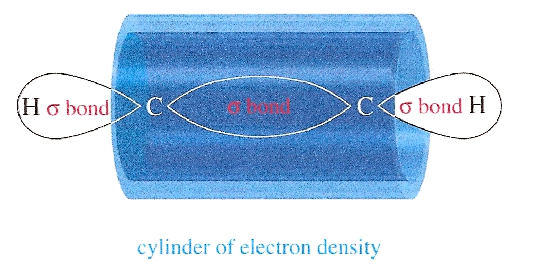
The H-NMR spectrum of 3,3-dimethyl-1-butyne shows a high field signal due to the alkynyl hydrogen on the terminal alkyne. This high field position suggests a relatively shielded hydrogen, which can be explained by the cylindrical electron cloud around the axis of the molecule. When the two ? bonds are subjected to an external magnetic field, these ? electrons will enter into a cylindrical motion that results in this strong shielding effect with high field chemical shifts, something that is absent in the alkenes. This electron cloud can be seen in the figure below.
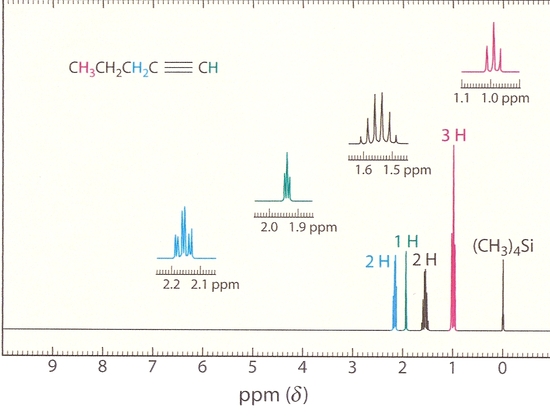
Another important aspect to keep in mind for alkyne NMR is the splitting of the peaks, or spin spin coupling. In 1-pentyne, for example, the terminal hydrogen is split by the hydrogens across the triple bond, even though it is separated from them by three carbons. As indicated in the figure below, the three peaks for the terminal hydrogen can be explained by this long range coupling, where the -CH2 group adjacent the sp carbon splits the terminal hydrogen.
Alkynes and Infrared Spectroscopy
Infrared Spectroscopy can be helpful in identifying terminal and internal alkynes.
Terminal Alkyne: Internal Alkyne:
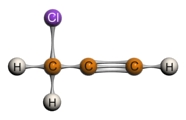
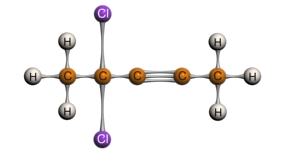
3-chloro-1-propyne 4,4-dichloro-2-pentyne
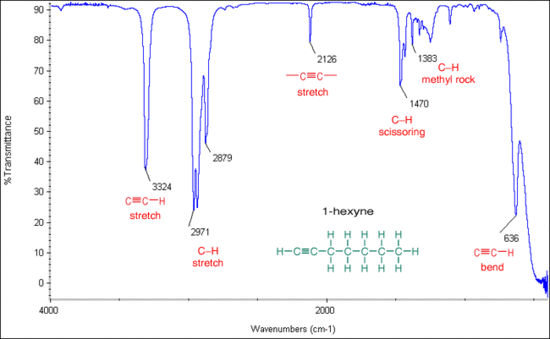
Internal alkynes, with its sp carbons attached to other carbons, will show weak bands for its triple bond at the 2100-2260 cm-1 region. However, this stretch is relatively weak, and is sometimes not present at all if the internal alkyne is symmetrical. In these cases, the IR spectrum loses its value as a helpful tool. Terminal alkynes, where the sp carbon is attached to a hydrogen, will show bands on the IR spectrum for both its alkynyl hydrogen and its triple bond. The C-H stretch on the terminal alkyne tends to appear as a strong, narrow band in the 3260-3330 cm-1 region while the triple bond shows a weak peak at 2100-2260 cm-1. Additional C-H bends will appear between 610-700 cm-1. The figure below shows these different characteristic stretches for internal and terminal alkynes.
References
- Graham, Patrick. Organic Chemistry. Boston: Taylor & Francis, 2004.
- Smith, Bryan. Infrared Spectral Interpretation. New York: CRC Press, 1999.
- Vollhardt, Peter, and Schore, Neal. Organic Chemistry: Structure and Function. New York: W.H. Freeman Company, 2007.
Outside Links
Problems
Based on the NMR and IR provided, what is the structure of C6H10?
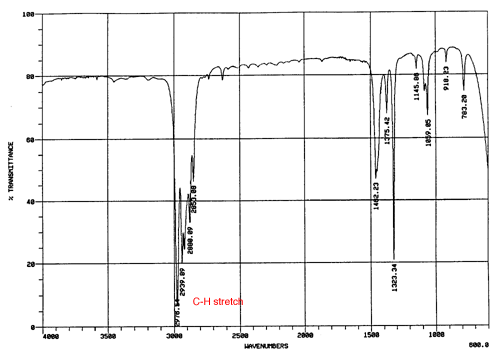
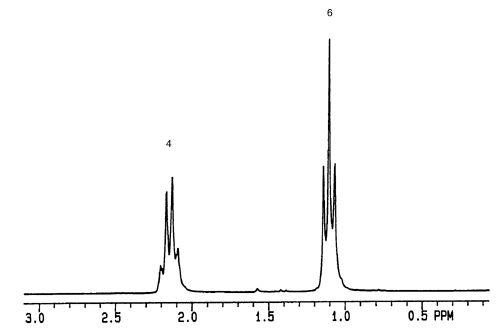
Answer: The degree of unsaturation is 2, indicating that the structure contains an alkyne, a triple bond. Because we don't see a peak at the 2100-2260 cm-1 range on the IR spectrum as expected, and a C-H stretch for an internal alkyne at 3260-3330 cm-1 is also absent, we can assume that a symmetrical internal alkyne is present. The NMR spectrum is more useful for this problem, and indicates that there are two equivalent -CH3 groups and two equivalent -CH2 groups. With this information, we get a molecule that looks like this:
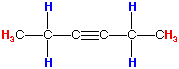
hexyne
Therefore, using both the IR and NMR spectrum, we get hexyne.
Contributors
- Rebecca Shragge

