4.7: Oxidative Addition- General Ideas
- Page ID
- 5072
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A critical difference between the transition metals and the organic elements is the ability of the former to exist in multiple oxidation states. In fact, the redox flexibility of the transition metals and the redox obstinacy of the organic elements work wonderfully together. Why? Imagine the transition metal as a kind of matchmaker for the organic elements. Transition metals can take on additional covalent bonds (oxidation), switch out ligands (substitution), then release new covalent bonds (reduction). The resulting organic products remain unfazed by the metal’s redox insanity. Talk about a match made in heaven!
The following series of posts will deal with the first step of this process, oxidation. More specifically, we’ll discuss the oxidation of transition metals via formal insertion into covalent bonds, also known as oxidative addition (OA). Although we often think of oxidative addition as an elementary reaction of organometallic chemistry, it is not an elementary mechanistic step. In fact, oxidative addition can proceed through a variety of mechanisms. Furthermore, any old change in oxidation state does not an oxidative addition make (that almost rhymes…). Formally, the attachment of an electrophile to a metal center (e.g., protonation) represents oxidation, but we shouldn’t call this oxidative addition, since two ligands aren’t entering the fray. Instead, we call this oxidative ligation (OL).
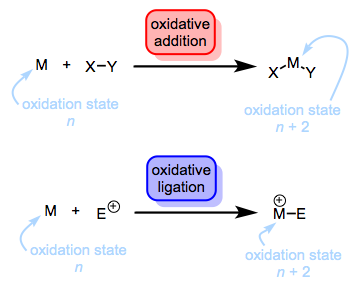
Protonation is (formally) a kind of oxidation. Who knew?! SN2 reactions with the metal as nucleophile are also oxidative ligations. Of course, if the leaving group comes back and forms a new bond to the metal, we’re back to oxidative addition. Both reactions lead to an increase in the oxidation state of the metal by two units and a decrease in the d electron count of the metal by two electrons. However, note how the total electron count changes in each case. The total electron count does not change during an oxidative ligation. Think of it this way: the new ligand brings no electrons with it to the complex. On the other hand, the total electron count of the complex actually increases by two electrons during oxidative addition. As a result, eighteen-electron complexes do not undergo oxidative addition. Carve that sucker on a stone tablet. Seventeen-electron complexes can undergo oxidative addition via bimolecular OA reactions, which leave X on one metal center and Y on another.
What are the mechanisms of oxidative addition, anyway? Let’s begin with the “concerted” mechanism, which can be thought of as σ-complex formation followed by insertion. The metal first sidles up to the X–Y bond and a σ complex forms (ligand dissociation may be required first). As we’ve seen, σ complexes M(X2) are tautomeric with their M(X)2 forms. When back donation from the metal is strong enough, the σ complex disappears and M(X)2 is all that remains. The metal has been formally oxidized: oxidative addition!

There are several variations on this theme. When X and Y are different, the σ complex is skewed and approach to the metal “asynchronous.” When the metal isn’t a great nucleophile, the reaction may stop at the σ-complex stage.
Other mechanisms of oxidative addition require multiple steps and the formation of polar or radical intermediates. An important two-step, polar mechanism involves SN2 attack of a nucleophilic metal on an electrophile, followed by coordination of the leaving group to the metal center. What we might call SN1-type mechanisms, involving dissociation of the electrophile before nucleophilic attack by the metal, also occur (HCl and other strong acids operate like this). Finally, both non-chain and chain radical mechanisms are possible in reactions of metal complexes with alkyl halides. We’ll dive into these mechanisms in more detail in upcoming posts.
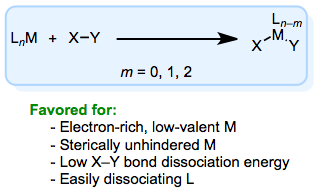
Hopefully from this general discussion, you’ve gleaned a few trends. The metal must have a stable oxidation state two units higher than its current OS for oxidative addition to occur. For the reaction to work well, the metal typically needs to be electron rich (and in a relatively low oxidation state) and the organic compound needs to be electron poor. To see why, consider that during oxidative addition, the metal formally loses two delectrons. Furthermore, the main-group atoms X and Y gain electron density, since the new M–X and M–Y bonds are likely polarized toward X and Y. The metal needs to bear two open coordination sites (not necessarily at the same time) for oxidative addition to occur, because two new ligands enter the metal’s coordination sphere. Since the new ligands need space, steric hindrance tends to discourage oxidative addition. Oftentimes ligand dissociation is required before oxidative addition can occur; in many of these cases, the rate of dissociation influences the overall rate of the reaction.