2. Metal Packing: Layers
- Page ID
- 4089
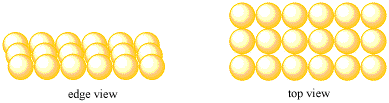
For comparison, maybe there is another set of atoms, also in a simple square layer. Suppose they are well-separated from each other; maybe they are far enough apart that you could fit an extra atom between each pair if you wanted to. If the free electron is in the same place -- the middle of the nearest hole -- you can see that it is much farther from the nucleus in this case. The force of attraction is much lower in this case, and the overall energy is not as low.
- Most metals pack very efficiently together to form a solid.
- Efficient packing leads to stronger bonding interactions.
That first case, with atoms packed more tightly together, may be preferable, because of the stronger interaction between the metal nucleus and the free electron. For reasons like this, understanding the packing efficiency in a crystal can be very important.
Contributors and Attributions
Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John's University)


