Answers to Chapter 06 Study Questions
- Page ID
- 11313
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
-
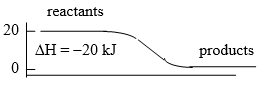
- exothermic, reactants
- exothermic, reactants
- endothermic, products
- exothermic, reactants
- exothermic, reactants
- endothermic, products
-
- \(\mathrm{\Delta H_f^\circ(C_3H_8) = -103.8\: kJ/mol}\)
- \(\ce{3 C(s) + 4 H2(g) \rightarrow C3H8(g)}\) \(\mathrm{\Delta H = -103.8\: kJ}\)
- exothermic
- \(\mathrm{1090\: kJ \times\dfrac{4\:mol\:H_2}{103.8\:kJ}=42.0\:moles\:H_2}\)
- \(\mathrm{30.1\:g\:C_3H_8 \times\dfrac{1\:mol\:C_3H_8}{44.0\:g\:C_3H_8}\times\dfrac{103.8\:kJ}{1\:mol\:C_3H_8} =71.0\: kJ}\)
- \(\ce{C3H8(g) + 5 O2(g) \rightarrow 3 CO2(g) + 4 H2O(l)}\)
- \(\mathrm{\Delta H(reaction) = 3 \Delta H_f^\circ(CO_2) + 4 \Delta H_f^\circ(H_2O) -\Delta H_f^\circ(C_3H_8)}\)
\(\mathrm{=3 (-393.5\: kJ) + 4 (-285.8\: kJ) - (-103.8\: kJ)}\)
\(\mathrm{= -1180 + (-1143) + 103.8 = -2220\: kJ}\)
-
- from the \(\mathrm{\Delta H_f^\circ}\) Table:
- \(\mathrm{\dfrac{1}{2} N_2(g) + \dfrac{1}{2} O_2(g) \rightarrow NO(g)}\) \(\mathrm{\Delta H = + 90.4\: kJ}\)
- \(\mathrm{\dfrac{1}{2} N_2(g) + O_2(g) \rightarrow NO_2(g)}\) \(\mathrm{\Delta H = +33.9\: kJ}\);
\(\mathrm{-2 \times (i) =}\) \(\mathrm{2 NO(g) \rightarrow N_2(g) + O_2(g)}\) \(\mathrm{\Delta H = -2(90.4) = -180.8\: kJ}\)
\(\mathrm{2 \times (ii) =}\) \(\ce{N2(g) + 2 O2(g) \rightarrow 2 NO2(g)}\) \(\mathrm{\Delta H = 2(33.9) = 67.8\: kJ}\);
\(\mathrm{overall\: reaction = 2 NO(g) + O_2(g) \rightarrow 2 NO_2(g)}\) \(\mathrm{\Delta H = -180.8 + 67.8 = -113.0\: kJ}\)
Exothermic
- \(\mathrm{\dfrac{1}{2} N_2(g) + \dfrac{1}{2} O_2(g) \rightarrow NO(g)}\) \(\mathrm{\Delta H = + 90.4\: kJ}\)
-
- \(\mathrm{Pb(s) + 0.5 O_2(g) \rightarrow PbO(s)}\) \(\mathrm{\Delta H = -217.9\: kJ}\)
- \(\mathrm{3 Pb(s) + 2 O_2(g) \rightarrow Pb_3O_4(s)}\) \(\mathrm{\Delta H = -734.7\: kJ}\); therefore:
\(\mathrm{2 \times (ii) = 6 Pb(s) + 4 O_2(g) \rightarrow 2 Pb_3O_4(s)}\) \(\mathrm{\Delta H = 2(-734.7) = -1469\: kJ}\)
\(\mathrm{-6 \times (i) = 6 PbO(s) \rightarrow 6 Pb(s) + 3 O_2(g)}\) \(\mathrm{\Delta H = -6(-217.9) = +1307\: kJ}\)
\(\mathrm{overall\: reaction = 6 PbO(s) + O_2(g) \rightarrow 2 Pb_3O_4(s)}\) \(\mathrm{\Delta H = -1469 + 1307 = -162\: kJ}\)
Exothermic
- from the \(\mathrm{\Delta H_f^\circ}\) Table:
- \(\mathrm{Q\: (J) = specific\: heat\: \left(\dfrac{J}{g\: ^\circ C}\right) \times mass\: (g)\times \Delta T \:(^\circ C)}\); \(\mathrm{\Delta T = 19.23 - 24.78 = -5.55\: ^\circ C}\)
\(\mathrm{Q = 4.18 \dfrac{J}{g\: ^\circ C} \times 60.0\: g \times -5.55\: ^\circ C = 1390\: J}\)
\(\mathrm{1\:mole\:NH_4Cl \times \dfrac{1390\:J}{5.03\:g\:NH_4Cl}\times\dfrac{53.5\:g\:NH_4Cl}{1\:mol\:NH_4Cl}=14,800\:J=14.8\: kJ/mole}\)
Endothermic
- \(\mathrm{Q = 6485\: \dfrac{J}{^\circ C} \times 10.7^\circ C = 69,400\: J = 69.4\: kJ}\)
\(\mathrm{1\: mole\: C_2H_4 \times \dfrac{28.0\:g\:C_2H_4}{1\:mol\:C_2H_4}\times\dfrac{69.4\:kJ}{1.40\:g\:C_2H_4}= 1390\: kJ = 1.39 \times 10^3\: kJ}\)
- \(\mathrm{2 NH_3(g) + 3 N_2O(g) \rightarrow 4 N_2(g) + 3 H_2O(l)} \hspace{38px} \mathrm{\Delta H = -1010\: kJ}\)
\(\begin{align}
& \mathrm{3 N_2H_4(l) + 3 H_2O(l) \rightarrow 3 N_2O(g) + 9 H_2(g)} && \mathrm{\Delta H = 3(+317)\: kJ}\\
& \mathrm{N_2H_4(l) + H_2O(l) \rightarrow 2 NH_3(g) + \dfrac{1}{2} O_2(g)} && \mathrm{\Delta H = +143\: kJ}
\end{align}\)
\(\underline{\mathrm{9 H_2(g) + 4\dfrac{1}{2} O_2(g)\rightarrow 9 H_2O(l)}\hspace{109px} \mathrm{\Delta H = 9(-286)\: kJ}}\)
\(\mathrm{4 N_2H_4(l) + 4 O_2(g) \rightarrow 4 N_2(g) + 8 H_2O(l)} \hspace{46px} \mathrm{\Delta H = -2490\: kJ}\)
for the reaction, \(\mathrm{N_2H_4(l) + O_2(g) \rightarrow N_2(g) + 2 H_2O(l)}\), \(\mathrm{\Delta H = \dfrac{(-2490)}{4} = -623\: kJ}\)