2.3: Types of Bonds
- Page ID
- 95808
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Metallic Bonds
Metals as either elements or alloys, which are mixtures of metals, have distinct properties. Metals are highly conductive of heat and electricity. They are dense, have high luster (shiny), metallic colors (copper, silver, and gold), can be pulled into wires (ductile), and can be beaten into sheets (malleable). The special bonding of metals is responsible for many of these properties. Metals bond by sharing their valence electrons and some of the d-orbital electrons between cations of the metal. These shared, freely-moving electrons are called a “sea of electrons” and is how metallic compounds and elements bond as liquids and solids.
Covalent or Molecular Compounds
Most compounds form when elements share valence electrons to form a covalent bond. Covalent compounds include organic compounds, which are compounds formed by living matter (for example, DNA, sugars, vitamins, and proteins), medicines, plastics and polymers, compounds that are gases and liquids at normal temperatures and pressures, acids, and many others. Most covalent compounds are combinations of nonmetallic elements, and may have hundreds even thousands of elements bound together. The smallest individual set of atoms making up each compound that has the properties of that compound is called a molecule.
Sharing of Electrons
Ionic bonding takes place by creating ions with filled s and p-orbitals, and sharing of electrons will take place until the atoms reach the same stable electron configuration. For example, each chlorine atom has 7 valence electrons, by sharing 1 valence electron each chlorine will have 8 (sharing electrons does not take away electrons it only adds electrons). Just like ionic compounds, the covalent compounds will have 8 valence electrons around each atom to create a stable electron configuration.
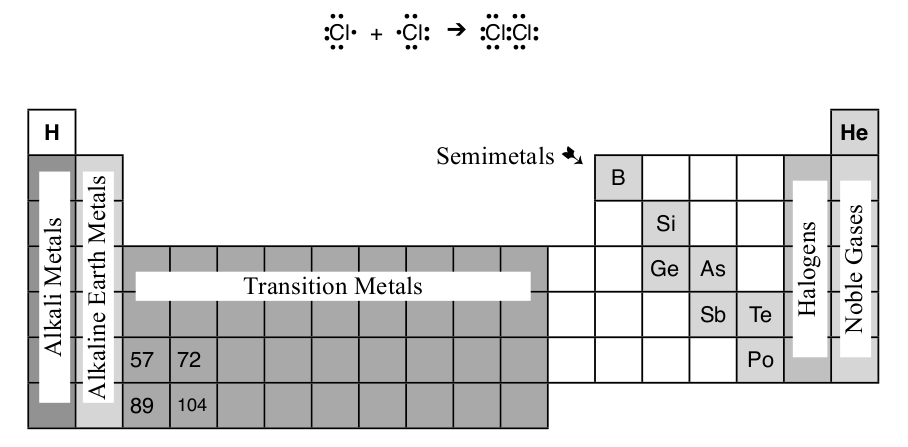
Diatomic Elements
A set of elements, which includes chlorine, form molecules of two atoms at normal temperature and pressures: H2, O2, N2, F2, Cl2, Br2, & I2. This group of elements include the halogens, hydrogen, oxygen, and nitrogen and can be recognized because they are the only elements that have names ending with -gen or -ine.
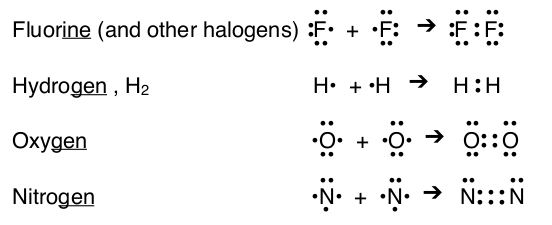
Notice that hydrogen has only 2 valence electrons around each atom after sharing. This is because only the first energy level is filled, which only requires two electrons.
Multiple Bonds
For oxygen and nitrogen, the atoms must share more than one electron to reach an octet for each atom. When two atoms share two pairs of electron then a double bond forms; when two atoms share three pairs of electrons then a triple bond forms. A single bond forms with only one shared pair of electrons Notice that for many simple examples of sharing the number of lone electrons an atom has equals the number of shared electrons in the compound. Unlike ionic compounds, most covalent compounds have more than two atoms. When three, four, five, and more atoms combine they share electrons until each atom reaches an octet (although there are exceptions).
| Elements | Electron Dot Diagram | Molecule | Name of Compound |
|---|---|---|---|
| C & H | 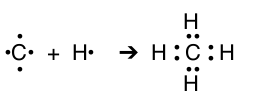 |
CH4 | Methane |
| N & H | 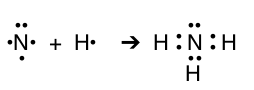 |
NH3 | ammonia |
| O & H |  |
H2O | water |
| C & Cl | 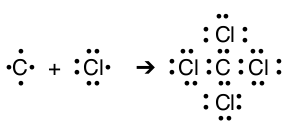 |
CCl4 | carbon tetrachloride |
| C & O |  |
CO2 | carbon dioxide |
| P & F |  |
PCl3 | phosphorus trichloride |
| H & C & N |  |
HCN | hydrogen cyanide |
Bonding electrons can be shown as lines connecting atoms and lone pair electrons can be shown as lines above an atom (http://commons.wikimedia.org/wiki/File:H2O.svg#file):

Hydrogen bonds
Hydrogen bonds are the strongest intermolecular bond (not inter- is a prefix meaning between). Hydrogen bonds only occur with a select group of atoms. When HF, HOR, or HNR 2 , where R is H or more of the molecule, are present then H bonding can occur with a O or N in a molecular compound or with HF. The double helix structure of DNA is due to the hydrogen bonds between the nucleotides. Water also has hydrogen bonds which accounts for many of its unique properties.

Hydrogen bonds in DNA (the dotted lines)
http://commons.wikimedia.org/wiki/File:DNA_chemical_structure.svg
Dipole-dipole
Dipole-dipole forces are the next strongest intermolecular forces. Polar molecules have shifted electron density that creates partially positive and partially negative charges at opposite ends of the molecule. The molecules will attract each other with the positive end attracting the negative end and visa versa. Unlike ionic compounds the molecules have strong bonding forces in the molecule but the intermolecular forces are easily broken.

These diatomic (two atom) molecules have dipole-dipole attractions. Because of the high electronegativity of one of the atoms the electron density has shifted to create a partial negative charge, δ–, (on top on the first molecule) and partial positive charge, δ+. Intermolecular bonding occurs through attraction of the positive and negative charges.
http://commons.wikimedia.org/wiki/File:Polare_atombindung.png
London or Van der Waals Forces
The weakest intermolecular forces are London Forces, also called Van der Waals forces. This intermolecular force is due to temporary dipoles. Since electrons are in constant motion each nonpolar molecules will form positive and negative end over time (the bonding electrons will randomly show up all on one side). A nearby nonpolar molecule will adjust its electron density because negative charges repel and positive charges attract electrons. This induces a dipole in a neighboring molecule so the two have an attraction. This is the mechanism for intermolecular attraction between nonpolar molecules.

Nonpolar molecules have evenly distributed electron density as shown by the first four molecules. But random motion can create a temporary dipole, the first molecule to the right of the arrow. The molecules near this temporary dipole react to the change in electron density and create their own dipoles; they have induced dipoles.
http://de.wikipedia.org/w/index.php?title=Datei:Unpolare_atombindung.png&filetimestamp=20070704231926
London forces are stronger when the valence electron are dispersed widely in large shells. This is because the electrons will create temporary dipoles that are more difficult to remove when the electrons are so distant from the nucleus and so dispersed in the large electron cloud. The most prominent example of the change in the intermolecular forces due to atomic size is the halogen family. Fluorine, F2, and chlorine, Cl2 are both gases. Like all halogens they are nonpolar because the difference in electronegativity is zero. As gases the molecules of the diatomic molecules do not attract each other and the molecules of gas move randomly and distribute themselves throughout the volume of their container. Bromine, Br2 , is a liquid; the only other liquid element at typical temperatures and pressures is mercury, Hg. Bromine has electrons filling the 4th energy level. The molecules of bromine have some attraction for each other because the substance is a liquid, in which the molecules move randomly, but they slide between molecules with small attractive forces holding the particles from separating to become a gas. Finally, iodine, I, , is a solid. The temporary dipole is longer-lived with the outer electrons in the 5th energy level and the molecules have longer, stronger attractions. (Some of the changes in the state of matter—from gas to liquid to solid—are due to the larger molecular weight, but temporary dipole changes have the most prominent role).
Differences in Attractive Forces
When molecules have strong intermolecular forces they are most likely to be solids. As solids every molecule is locked in place by the strong attractions. As the intermolecular forces are reduced the molecules may be liquids where the forces keep the particles near to each other, but the forces are not strong enough to stop random motion. Finally, weak intermolecular forces are present in gases which cannot maintain any hold on each other so they move randomly and in their own path filling the volume of their container. So water, H2O, is a liquid despite its small molecular mass, while CH4 , methane, is a gas (the natural gas used in stoves and water heaters). Remember that molecular weight has something to do with whether molecules are solids. Waxes and your skin are essentially nonpolar, but they are solid, because they have large numbers of atoms in each molecule (wax is C15H31CO2C30H61) so the molecules are massive and have long, long molecular orbitals so induced and temporary dipoles are long lasting.
Properties Affected by Intermolecular Forces
Intermolecular forces affect the physical changes that substances undergo and their state of matter at standard temperature and pressures. State of matter is determined by the type of intermolecular forces. The stronger the intermolecular force the more likely it is a solid or a liquid (generally, only molecules with high molecular masses will be a solid). Boiling point and melting point, the temperature at which a substance boils or melts, are also affected by intermolecular forces, with higher temperatures related to stronger intermolecular forces. Vapor Pressure, which is the pressure of the gas molecules of a pure substance that evaporate from a pure substance at a temperature below the boiling point, is higher for weaker intermolecular forces.
Contributors
Kenneth Pringle and Curriki. This content is licensed under a Creative Commons Attribution Share-Alike 3.0 License.

