2.0: Introduction
- Page ID
- 95668
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Unit 1 covered the atomic structure of elements and the periodic table of elements. There are only 92 elements that occur in natural, many of these is extremely rare, and 26 elements have been created in large, expensive experimental sites like the Stanford Linear Accelerator Center and the Large Hadron Collider in Geneva. The millions of other pure substances both in nature and created in labs are compounds. Compounds form when elements combine and their atoms held together by chemical bonds. This unit is about how compounds form and the properties of different types of compounds.
Ionization Energy and Electronegativity
Chemical bonds form when electrons are transferred or shared between atoms. The periodic trends, of ionization energy and electronegativity, help explain why metals and nonmetals react by transferring electrons and two nonmetals will share electrons when bonding. Ionization energy is a measure of how hard it is to remove an electron. Metallic elements on the left of the periodic table have low ionization energies and lose electrons easily. Electronegativity measures the pull of the atomic nucleus for electrons towards itself. The nonmetals in the upper right with a high electronegativity will form bonds by sharing electrons between atoms of similar electronegativity as well as attract electrons from metallic elements with a low electronegativity.
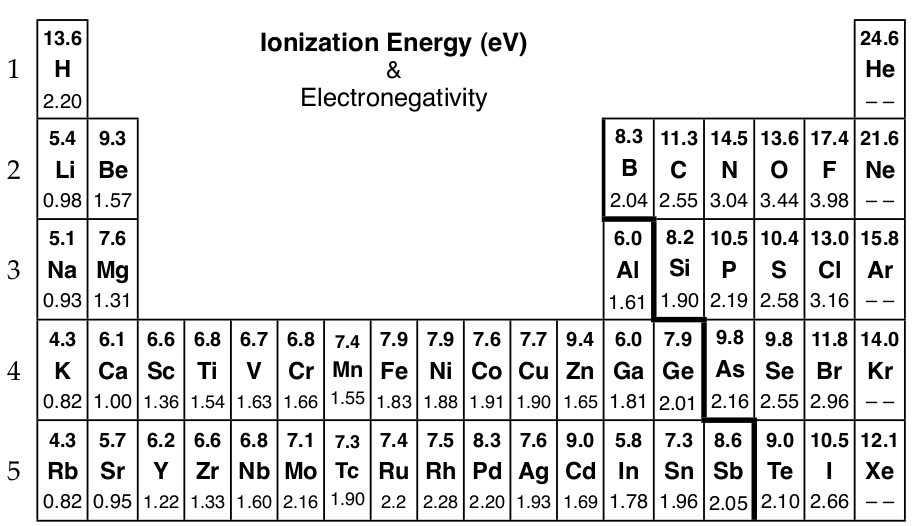
Ionization Energy (top value) and Electronegativity of Selected Elements
Transferring Electrons
Ionic compounds are made by transferring electrons to become ions. A cation, which is a positive ion, forms by losing an electron, and an anion, a negative ion, forms when the other element gains an electron. The two ions will attract each other because of their opposite charge by a bonding force called electrostatic attraction.
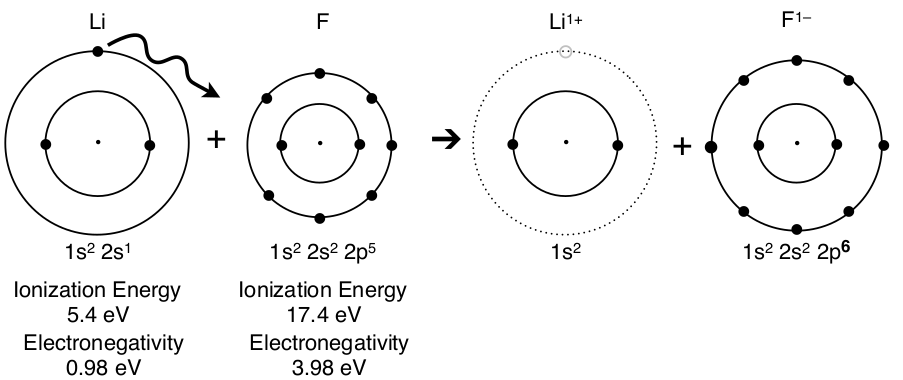
The Transfer of an Electron from a Lithium Atom to a Fluorine Atom
A lot of chemistry takes place in this diagram that common with most synthesis reactions that combine elements to make an ionic compound. Let’s start with the reaction. The two atoms at the left are called the reactants, which are the starting materials of the reaction, and the two ions at the right are called the products, which are the results of a reaction. The arrow, ➔, means yields, forms, gives, reacts to make, etc. The circles in the diagram represent the energy levels or shells of the electron configuration and each dot, •, is an electron. This is called the Shell Model and can be a valuable tool to show the movement of electrons and the formation of chemical bonds. Notice that the electron configuration given below the shell model provides the same information about the number of electrons on each shell.
The low ionization energy of lithium, Li, shows that it takes little energy to remove the electron. The ionization energy of fluorine is higher and fluorine will not lose its electron. The electronegativity of fluorine is high (the highest value actually) and its nucleus has a high attraction for electrons. These two factors combine to cause the transfer of an electron to fluorine. The products of the reaction are a lithium cation, Li1+ , which has lost a negative electron while not changing the three positive protons in the nucleus so it has a positive charge, and a fluorine anion, F1–, which has an extra electron so a negative charge. The superscript, 1+ and 1–, represent the charge of the ion and the number of electrons lost (for +) and electrons gained (for –).
Finally, it is important to remember that lithium has a larger radius than fluorine since fluorine has a greater effective nuclear charge (1 shell shielding the valence electrons and the nucleus changes from +3 to +9). This is represented in the shell model. But when fluorine gains an electron there are more electron-electron repulsions and the +9 charge is distributed among ten electrons instead of nine so the radius of the anion increases. Likewise the lithium cation is much smaller than the lithium atom, since the electron in the outer shell is gone and the inner shell experiences an unbalanced positive charge (nucleus is +3 and electrons are -2).
Noble Gas Electron Configuration is Stable
In the reaction between lithium and fluorine only one electron was transferred, but as many as four electrons can be transferred in common reactions. The valence electrons, which are the outermost electrons, are most easily removed because of shielding from the inner electrons. In the figure below each atom from lithium to carbon shows the same result when all the valence electrons are removed: two electrons in a 1s 2 electron configuration, which is the same electron configuration of helium.
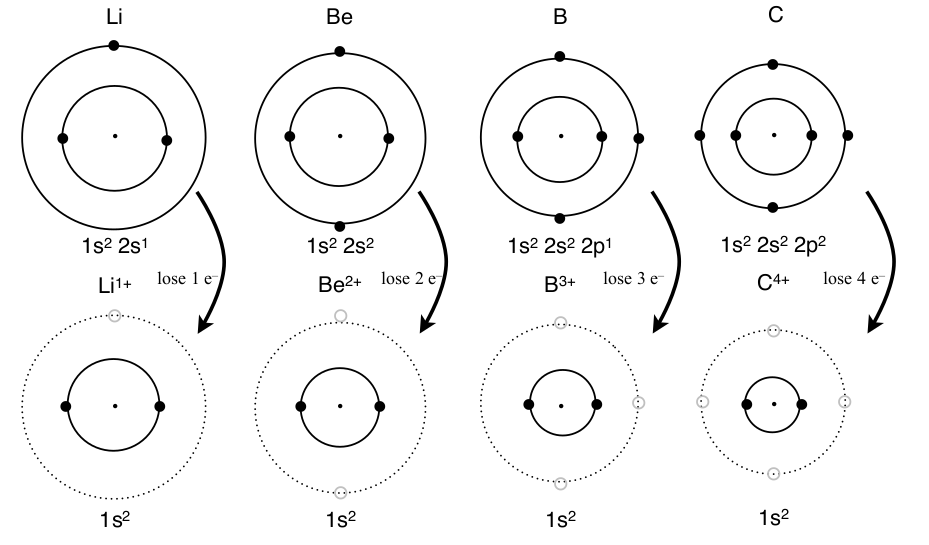
Second Period Metals Losing Electrons to Form a Stable Noble Gas Electron Configuration
Metals with low ionization energies lose electrons until the valence electrons are removed. No inner electrons are removed in the reaction because they are close to the nucleus which has extra positive charge and removing these electrons would take too much energy compared to reacting with other atoms that still have valence electrons. Since it is so common for the valence electrons to be lost in a reaction and the inner electrons remain with s and p orbitals filled, noble gas electron configurations are considered stable.
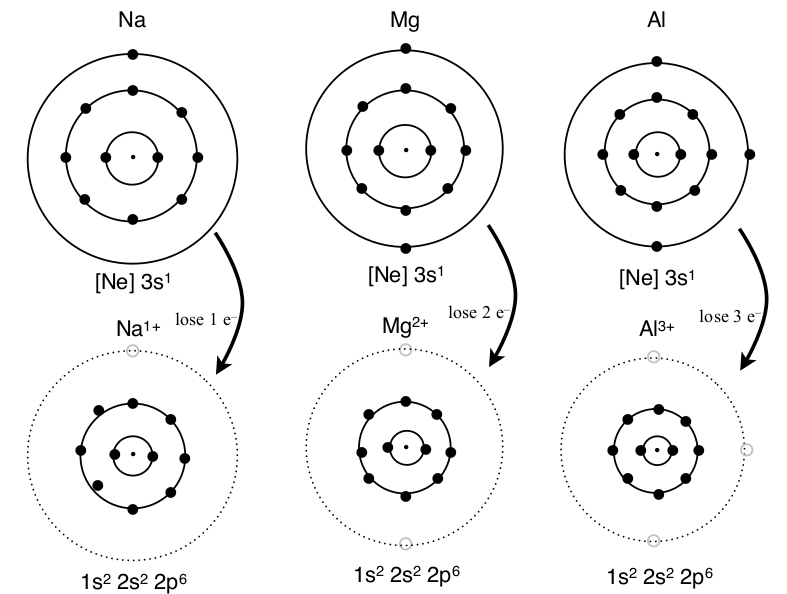
Third Period Metals Losing Electrons to Form a Noble Gas Electron Configuration
When atoms gain electrons, they also stop adding electrons when they get to a noble gas electron configuration, with a filled set of s and p orbitals, because gaining even more electrons would require a new, more distant energy level or shell to hold the electrons. The atoms that gain electrons have high values of electronegativity, which indicates the nucleus has a strong pull toward electrons. Nonmetals on the upper right of the periodic table, other than noble gases, have high electronegativities and form anions easily by gaining electrons.
Second Period Nonmetals Gaining Electrons to form Stable Noble Gas Electron Configurations
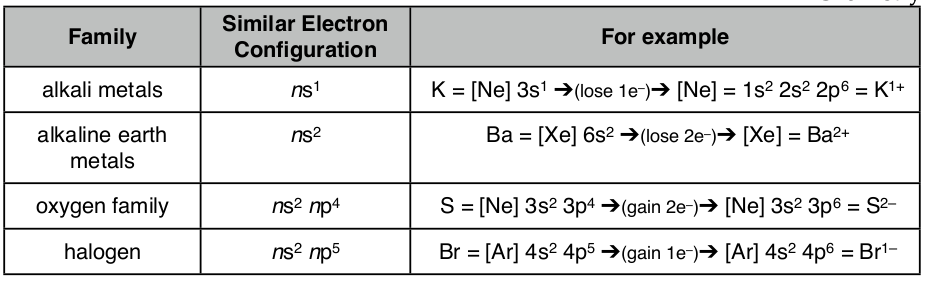
Because the electron configurations for families of elements are similar their chemical reactions are similar. For example, the alkali metals the electron configurations is ns1, where n is the highest energy level or shell. Thus, all alkali metals easily lose one electron in a reaction where there is a transfer of electrons. Likewise, alkaline earth metals with electron configurations of ns2 will lose two electrons. On the other side of the periodic table the halogens will gain one electron since their electron configuration is ns2np5 , while the oxygen family will gain two electrons because these atoms have electron configurations of ns2np4 .
Most elements gain or lose electrons until a noble gas electron configuration is reached because any further changes are energetically unfavorable. Gaining more electrons requires adding electrons to a more distant energy level and losing more electrons would remove electrons from inner energy levels. Transition metals, however, with their d-orbitals, usually have multiple types of ions (tin has Sn2+ and Sn4+). This is because the d–orbital electrons are in an inner shell, ns2(n – 1)dx, and have energies very close to the outer s-orbital electrons. So while the outer s-orbital electrons are usually removed, some d-orbital electrons can also be removed. For example, manganese, [Ar]4s23d5, can have cations of Mn2+, Mn3+, Mn4+, Mn6+, and Mn7+.
Contributors
Kenneth Pringle and Curriki. This content is licensed under a Creative Commons Attribution Share-Alike 3.0 License.


