1.5: Periodic Trends
- Page ID
- 95475
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Periodic Trends
We have already identified one trend on the periodic table: the change from metals to nonmetals as we move right across the groups. There are other periodic trends that show change in properties as you move across the groups or down the period. There are interactive periodic tables on the internet that allow you to pick properties and see the changes in color across the periodic table. Please visit : Dynamic Periodic Table, http://www.ptable.com, and Interactive Periodic Table, http://www.touchspin.com/chem/DisplayTable.html.
There are three major trends. Ionization energy which is the energy needed to remove an electron from an atom or an ion (an ion is an atom with electrons added or removed to give it a negative or positive charge respectively). Electronegativity which is a measure of the ability of an atom to pull electrons from another atom while the two are bonded. And atomic radius, or size, which is the 1⁄2 the distance between two atoms of an element that are bonded (less specifically, it is the radius of a single atom). The ionic radius is similar to the atomic radius, but every added electron increases the radius and every removed electron decreases the radius. Thus, all negative ions are larger than positive ions in the same period, or row, since positive ions have electrons removed.
Factors Influencing the Periodic Trends
The periodic trends have their basis in the interaction of charge. The positive protons in the nucleus are attracting, or pulling, on the negative electrons around it. Also the electrons are repelling each other so that inner electrons are pushing the outer electrons away and outer electrons are repelling each other to the farthest space.
Effective nuclear charge is the positive charge pulling the outer electrons towards the nucleus. The effective nuclear charge increases as you move across a period, since each atom is gaining a new proton in the nucleus. The term “effective” is used because adding one proton does not increase attractive force by +1 charge because of the shielding effect of inner electrons in an atom. For example, the effective nuclear charge for the second period is Li = 1.28, B = 2.58, C = 3.22, N = 3.85, O = 4.49, and F = 5.13. You can see that the effective nuclear charge is not the same as the total nuclear charge: +3 for Li to +9 for F. The effective nuclear charge is distributed over over the number of electrons in the atom
Shielding or Screening is a property of inner electrons reducing the attractive force of the nucleus on the outer electrons. Shielding increases as you move down a period. Since electrons repel each other, the inner electrons keep outer electrons farther away from the nucleus. Coulombs law says that the distance charged sources apart reduced the force between the sources by 1/r, where r is 1/2 the distance between the two sources. Thus as the electrons move away from the nucleus the force of attraction is reduced significantly. You may have experienced something similar when you move a magnet closer and closer to a metal surface all of a sudden the force increases drastically and the magnet moves against the surface it is attracted to in a little jump. So if the outer electrons are pushed away then the attractiveness is reduced in a measurable amount.
Ionization Energy a Proof of the Quantum Model of Electron Arrangement
Ionization energy is the energy needed to pull an electron away from an atom. Ionization energy is highest for helium, He and lowest for francium, Fr.
Ionization Energy highlighting Alkali Metals and Noble Gases. https://commons.wikimedia.org/wiki/File:IonizationEnergyAtomicWeight.PNG
The figure above shows the trend in ionization. For families like alkali metals and noble gases, the ionization energy decreases moving down a group. This is because each time an element has a new energy level, or shell, added the electrons are farther away from the nucleus, these outer electrons are shielded by the inner electrons. Ionization energy also increases moving across a group from left to right. This is because the number of protons and the positive charge in the nucleus increases across the period, so the electrons are attracted more strongly to the nucleus (and there is no additional shielding because as you move across a period you don’t change inner electrons). A more interesting observation of the ionization energy graph is how it mirrors the quantum theory of electron arrangement. Noble gases all have the highest ionization energy in their period and the very next element, which is an alkali metal, has a lower ionization energy about 2 to 4 times smaller. This observation matches our model of a new larger electron shell being started for the alkali metals.
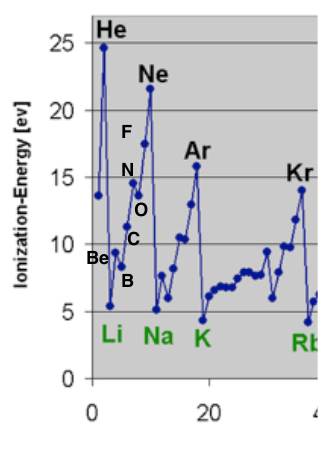
Furthermore, the ionization energy from Li to Be increases but for B decreases a bit. This confirms the change from the s-orbital to p-orbital and the higher energy of a p-orbital which would make the electron easier to remove. Then the ionization energy increases from B to N as more protons in the nucleus increase the attractive force and make the electrons more difficult to remove. But for N to O there is another decrease in ionization energy. This matches our theory that the extra electron in oxygen (compared to nitrogen) is placed in an orbital containing another electron and the repelling force between the two electrons in the same orbital reduces the ionization energy. Finally, we can see the long flattened area of the graph to the right of K, potassium, which is the d-orbital electrons. Placed beneath the 4s electrons, the 3d electrons provide shielding that cancel out the added proton to the nucleus, and the valence electrons in 4s have the same ionization energy across the transition metal.
Electronegativity
Electronegativity, which is a measure of the attractive force of an atom’s nucleus for the electrons that are shared in a bond with another atom, follows the same trend as ionization energy. Electronegativity is highest in the upper right with F, fluorine and lowest in the lower left with Fr, francium. However, since noble gases are unreactive and they don’t bond with another atom usually, there is no electronegativity for He, Ne, and Ar.
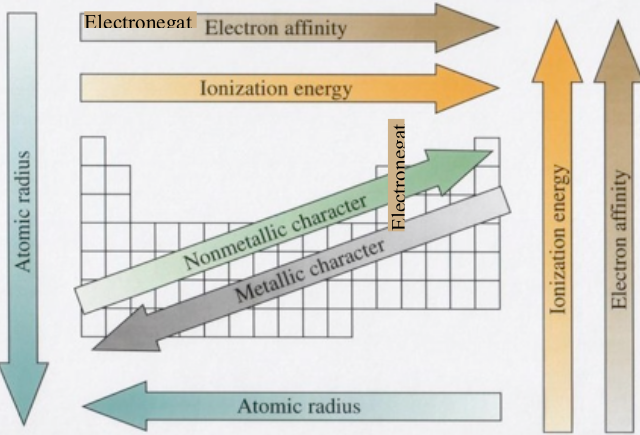
Periodic Trends
modified from http://commons.wikimedia.org/wiki/File:PERIODIC_TRENDS.jpg
Atomic Radius or Atomic Size
The atomic radius of an atom will be smaller when there is a greater positive charge in the nucleus, there is fewer electron-electron repulsive forces (shielding), and the number of energy levels, or shells, is greater. Thus, the trend for atomic radius is opposite to the trend for ionization energy; the atomic radius increases as you go down a group (adding energy levels) and increases from right to left across a period.
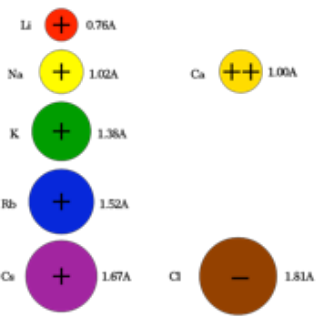
The alkali metal ions increase in size down the group. Ionic size inreases from right to left, as Ca 2+ is smaller than Na 1+ . But gaining electrons increases the size of atom, so Cl 1– , which is in the same period as Ca 2+ and Na 1+ , is much bigger than either although it is on the right. However, Cl 1– is smaller than O 2– the next ion to its right.
Ionic Radius or size of ion
The ions of elements form when a chemical reaction causes an electron to be added or removed from a neutral element (neutral is when the number of electrons and protons are equal). Ions that gain a electrons have an increased radius, since electron-electron repulsions increase and the attractive force of the nucleus is distributed over a larger number of negative charges. Ions that lose electrons on the other hand will shrink because there are fewer repulsive forces and the positive, attractive force acts on fewer negative electrons. So while the trend in ionic size is the same as for atomic size (increasing size moving down and to the right), the ions that have gained electrons (called anions), are always larger than the ions that have lost electrons (called cations).
Contributors
Kenneth Pringle and Curriki. This content is licensed under a Creative Commons Attribution Share-Alike 3.0 License.


