1.2: Atomic Models
- Page ID
- 95470
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Atomic Model
The modern model of the atom is built from bits and pieces that scientists have added to the study of chemistry. Important names like Thompson, discoverer of the electron, Chadwick, discoverer of the neutron, Millikan, discoverer of the charge of the electron, and Mosely, who figured out how to determined the atomic number in 1903, are given this brief mention though their contributions where enormous. There are many others and you are encouraged to discover for yourself the rich history behind the present day atom. So while the atomic model was developed after a series of hypothesis and theories were put forward, reworded, replaced as new experiments required new theories. What we know today as certainty, may change if new tools are developed that are sensitive enough to make the keen observations necessary to fully support new theories (see http://www.neoam.cc.ok.us/~rjones/Pages/online1014/ chemistry/chapter_8/pages/history_of_atom.html and listen to http://uh.edu/engines/epi2237.html )
Gold Foil Experiment
The one experiment that provided the strongest evidence for the nuclear model of the atom is Rutherford’s Gold Foil Experiment. At Rutherford’s labs in Cambridge, England, they set up an experiment where a radioactive particle, an alpha particle, is fired at a very thin sheet of gold. The expectation of is that the particles will fly though the thin gold foil, because it is thought that gold’s mass is distributed throughout its volume (the alpha particle is dense and small like a bullet and the foil is like paper). But much to the astonishment of the researchers some particles are reflected back away from the foil while most make their way through the foil. This scenario made it impossible to use a model where the atom was a solid sphere like a marble. After much contemplation (and repeating the experiment), Rutherford theorized that the atom must have a small, dense, and positive nucleus. This would explain the small number of particles that bounced back, 1 in 8800, and why the nucleus wouldn’t fall apart with the collisions. This was the beginning of our understanding of the modern, nuclear atom.

The Gold Foil Experiment
https://commons.wikimedia.org/wiki/File:Gold_foil_exp_conclusions.jpg
The modern atom has a small dense nucleus in the center of a sphere. This nucleus is 10,000 times smaller than the outer boundary of the atom, but it must contain nearly all the mass of the atom. This is an astounding notion. For example, imagine a two story house that weighs 100,000 pounds, but instead of the mass being contained throughout the house it is all located in a pea dropped in the middle of the second floor. So you can lift up all the parts of the house because they have very little mass, but when you get to the pea it won’t budge, because it weighs as much as the entire house.

The volume around the nucleus, called the electron cloud, is filled with electrons that are moving near the speed of light (about two million meters per second– http:// www.newton.dep.anl.gov/askasci/phy99/phy99092.html ). These electrons move randomly in different directions and different speeds. 90% of the time the electrons are within the atom’s radius. And like some super freeway the electrons are speeding so fast nothing will cross their path. This is how atoms have outer boundaries that can be measured.

Parts in the Atom and their Size
Fundamental Particles in the Atom
The proton is located in the nucleus and has a mass that is the nearly identical to neutron’s and 1836 times bigger than an electron. The atomic number of an element is equal to the number of protons and differs for each element. A proton has a positive charge. The neutron is also in the nucleus and has a neutral charge. When the number of neutrons changes the new atom is an isotope. Isotopes are atoms with the same number of protons but different number of neutrons. Another definition is that isotopes are atoms of the same element with different mass numbers. The electrons are negative particles with very little mass and travel throughout the nucleus’s volume. The number of electrons in a neutral atom is equal to the number of protons. The electrons have a negative charge with very little mass. Electrons are also responsible for the chemistry of substances because the nucleus is so deeply buried in the core of the atom
|
Name |
Location |
Relative Mass |
Charge |
| Electrons | Electron clouds | \(\frac{1}{1836}\) | negative , e- |
| Proton | nucleus | 1 | positive, p+ |
| Nuetron | nucleus | 1 | nuetral, n0 |
Quarks
Very recently scientists discovered smaller pieces of protons and neutrons called quarks (1968 to 1995— http://particleadventure.org/other/history/smt.html). Quarks and electrons seem to be the smallest particle that makes up matter. Three quarks are needed to make up both a proton and a neutron, but a proton is made up of two “up” quarks and one down quark so that the total charge is +1, and the neutron has two down quarks and 1 up quark so that the charge is neutral (up quarks have a charge of \(+\frac{2}{3}\) and a down quark has a charge of \(-\frac{1}{3}\)).111
Charge
A word about charge. Charge is a unique property that exerts a force between charged matter. If matter has the same charge then there is a pushing force between the objects. If the substances have opposite charge then the substances are attracted (“like charge repels, unlike charges attract”). But since protons are stuck in the nucleus and cannot move (changing protons changes the element), positive matter is created when electrons are lost and negative matter is created when electrons are gained. The static electricity of a balloon caused by rubbing it against a fuzzy shirt or hair is a property of charge. The balloon gains electrons from the fuzzy clothing and has a negative charge. The shirt or hair is positively charged with the lost electrons. The oppositely charged balloon and hair stick to each other with an attractive force. This is a very strong force, since we move only relatively few electrons and yet overcome the force of gravity . Charge is important in understanding the bonding taking place within and between elements, as well as, the interaction between electrons and between electrons and the nucleus.

Balloons and hair show electrons move to create static charge
http://www.flickr.com/photos/jemsweb/2903099055/in/photostream/
Determining the Number of Fundamental Particles in a Neutral Atom
The number of protons in any atom of an element is equal to the atomic number. The number of electrons is equal to the number of protons. This makes the atom neutral, which is when the sum of positive and negative charges cancels out.
Atomic Mass and Neutrons
The atomic mass of an element is the combination of the protons and neutrons found in the nucleus of the atom. For fluorine with an atomic number of 9 and atomic mass of 19.0 amu, the number of protons is 9 and the number of neutrons is 10 (since 19 = 9 + 10). For chromium the atomic number is 24 and the atomic mass is 52.0 amu (amu is the unit for mass for an atom that means atomic mass unit). So the number of protons for chromium is 24 and the number of neutrons is 28 (since 52 = 24 + 28). But how does this work for something like boron, B, with an atomic mass of 10.80 or for chlorine, Cl, with an atomic mass of 35.45. Atomic mass is actually a calculated number based on the different isotopes of an element. Isotopes are atoms of the same element with different number of neutrons. For example boron there are two major isotopes,, boron-10 and boron-11. Boron-10 has 5 protons and 5 neutrons and boron-11 has 5 protons and 5 neutrons. The 10 in boron-10 is called the mass number. Since fluorine and chromium a single isotope that is 100% of the atoms, then the calculation is not needed.
The calculation to determine atomic mass is called a weighted average. For example, boron has two major isotopes, boron-10 and boron-11, and all natural samples of boron contain 20% boron-10 and 80% boron--11. The average mass of the atoms in a natural sample is calculated by: 0.20 • 10 + 0.80 • 11 = 10.8 amu (a more exact calculation of mass would use the actual mass of the isotope). Here is another example using chlorine. Chlorine also has two major isotopes: chlorine-35 and chlorine-37 with natural abundances of 75% and 25% (natural abundance is the percent of an isotope found in nature). So this weighted average calculation is: 0.75 • 35 + 0.25 • 37 = 35.5.
Electron Configuration and the Periodic Trends
Our model of the atom has the electrons on the outer boundary and in this location they will be the particles that interacts with other substances in chemical reactions. But we also know from the groups on the periodic table (alkali metals, alkaline earth metals, halogens, and noble gases) that the same reactivity and properties reoccur as more electrons are added. This means that adding electrons (recall electrons equal the protons in a neutral atom) does not gradually change the properties of atoms, but rather properties of atoms repeat as electrons increase. Therefore, the arrangement of electrons changes across the periodic table (to change properties) and then the arrangement returns to something similar so that the properties of elements repeat in the families of elements. Thus, the origin of an elements chemistry must lie in the arrangement of electrons.
The Bohr Model of Electron Configuration

The arrangement of electrons in the atom also needs a theory that predicts and explains the properties. Many of the early theories suggested that the electrons traveled like planets in orbits about the nucleus. The problem with this theory was that it was known that an object traveling around an attractive center will slowly move towards the center. So if the orbit theory was true then some atoms would be spontaneously changing as electrons and protons reacted when when the electron spiraled into the attractive, positive nucleus. Below is a brief description of the quantum model of the atom. you are encouraged to look at these two sources for a complete description: Physics 2000 at http://www.colorado.edu/ physics/2000/atomic_lab.html and Hyperphysics at http://hyperphysics.phy-astr.gsu.edu/ hbase/bohrcn.html#c1 .
Neils Bohr suggested that electrons had to remain in specific energy levels that had fixed energies. This restriction forced electrons to maintain their energy. In support of this theory, he showed calculations of the emission spectra of hydrogen gas that matched his theory. Emission spectra is the release of light after energy has been added to a substance. Unfortunately, this was the only substance that Bohr’s model fit. Fortunately, Bohr was able to put together a number of ideas and theories and point the way to the model we use today.
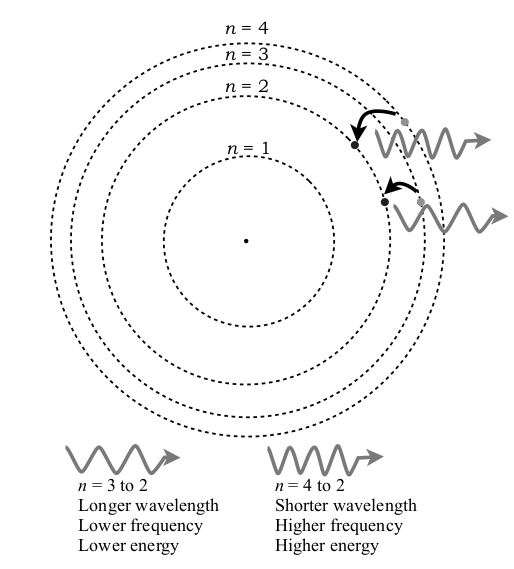
Bohr Model and Explanation
In the Bohr model, electrons can only travel to specific energy levels (labeled n = 1, 2, 3, etc.). An electron has more energy if it is farther away from the positive nucleus. In this figure, an electron (the outer dot) moves from a higher energy level, n = 3, to a lower energy level, n = 2. The electron must lose energy to move to the lower energy level so it gives off light (an individual light packet of energy is called a photon, and each changing electron gives off a photon). The greater the difference between the two energy levels, the greater the energy and frequency of light. A spectrum of an atom is created by all the different wavelengths of light released when energy is emitted. Light in the visible spectrum is a result of electrons relaxing to n = 2 from all the higher energy levels. Electrons that move to n = 1 will give off ultraviolet light, and electrons that move to n = 3 will give off infrared light.
Mathematics of Waves and Light.
The mathematics needed for a deep understanding of quantum theory is quite complex. Here are two simple, basic equations that show the relationship between the the energy of light emitted (or absorbed) by an atom and the energy difference between energy levels that the electrons travel in. Different types of light have different frequencies, ν. When you are changing radio channels you are changing frequencies that are emitted from each radio stations (light is all parts of the electromagnetic spectra which includes radio waves, microwaves, infrared waves, visible light, ultraviolet light, x-rays, and gamma rays). Another measurement of light is wavelength, λ. Frequency and wavelength are related to each other by the speed of light, c: c = νλ (the speed of light is a constant through a vacuum, 3 X 10 8 m/s). Energy of any frequency of light is calculated by using another constant, h. h is plank’s constant and equals 6.63 X 10 –34 m 2 kg/s. Plank’s constant is the smallest step of energy that can separate energy levels in an atom or between frequencies of light (on a normal scale energy is continuous because Plank’s constant is extremely small and differences in the steps are unobservable). Thus E = hν and has units of measurement of joules, J.

Hydrogen Emission Spectrum (Visible Light, Balmer Series)
http://commons.wikimedia.org/wiki/File:Visible_spectrum_of_hydrogen.jpg
These two equation were used by Bohr to support his model of the arrangement of electrons for the hydrogen electron. Energy is emitted as light when an electron loses energy to move to a lower level. But the change has to take place in steps of h, Plank’s constant. If the change was gradual or varied, then the bands of light would broaden and create a rainbow of light. Since the change has to be in steps, the lights appear as distinct lines. Each color is related to a wavelength, λ, or a frequency, ν. The frequency and wavelength of light is related to the energy, E = ν•h, needed to change energy levels. Comparing the observed light spectra with the energy predicted by theory, provides evidence for the correctness of the theory.
Schödinger and others showed that the behavior or electrons follows the pattern of waves better than as objects that obeying traditional physics like the movement of planets, which is what Bohr’s model was based on. They modified Bohr’s model using wave equations, or functions, to model the energy of electrons. The result is the quantum model, the Schödinger model, of the atom which correctly predicts the behavior of electrons and provides us with a physical representation of the location of electrons that we call electron configuration (electrons in compounds can also be modeled by quantum theory, but such models are much more complex).
Contributors
Kenneth Pringle and Curriki. This content is licensed under a Creative Commons Attribution Share-Alike 3.0 License.


