4.11: Cyclohexane
- Page ID
- 30349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Although the customary line drawings of simple cycloalkanes are geometrical polygons, the actual shape of these compounds in most cases is very different.
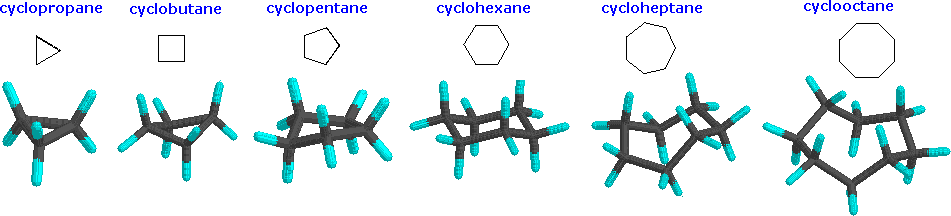
Cyclopropane is necessarily planar (flat), with the carbon atoms at the corners of an equilateral triangle. The 60º bond angles are much smaller than the optimum 109.5º angles of a normal tetrahedral carbon atom, and the resulting angle strain dramatically influences the chemical behavior of this cycloalkane. Cyclopropane also suffers substantial eclipsing strain, since all the carbon-carbon bonds are fully eclipsed. Cyclobutane reduces some bond-eclipsing strain by folding (the out-of-plane dihedral angle is about 25º), but the total eclipsing and angle strain remains high. Cyclopentane has very little angle strain (the angles of a pentagon are 108º), but its eclipsing strain would be large (about 10 kcal/mol) if it remained planar. Consequently, the five-membered ring adopts non-planar puckered conformations whenever possible.
Rings larger than cyclopentane would have angle strain if they were planar. However, this strain, together with the eclipsing strain inherent in a planar structure, can be relieved by puckering the ring. Cyclohexane is a good example of a carbocyclic system that virtually eliminates eclipsing and angle strain by adopting non-planar conformations. Cycloheptane and cyclooctane have greater strain than cyclohexane, in large part due to transannular crowding (steric hindrance by groups on opposite sides of the ring).
Conformations of Cyclohexane
A planar structure for cyclohexane is clearly improbable. The bond angles would necessarily be 120º, 10.5º larger than the ideal tetrahedral angle. Also, every carbon-carbon bond in such a structure would be eclipsed. The resulting angle and eclipsing strains would severely destabilize this structure. If two carbon atoms on opposite sides of the six-membered ring are lifted out of the plane of the ring, much of the angle strain can be eliminated.
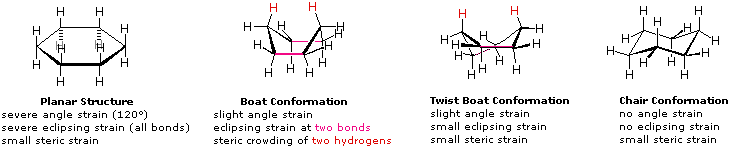
This boat structure still has two eclipsed bonds and severe steric crowding of two hydrogen atoms on the "bow" and "stern" of the boat. This steric crowding is often called steric hindrance. By twisting the boat conformation, the steric hindrance can be partially relieved, but the twist-boat conformer still retains some of the strains that characterize the boat conformer. Finally, by lifting one carbon above the ring plane and the other below the plane, a relatively strain-free 'chair' conformer is formed. This is the predominant structure adopted by molecules of cyclohexane.
Investigations concerning the conformations of cyclohexane were initiated by H. Sachse (1890) and E. Mohr (1918), but it was not until 1950 that a full treatment of the manifold consequences of interconverting chair conformers and the different orientations of pendent bonds was elucidated by D. H. R. Barton (Nobel Prize 1969 together with O. Hassel). The following discussion presents some of the essential features of this conformational analysis.
On careful examination of a chair conformation of cyclohexane, we find that the twelve hydrogens are not structurally equivalent. Six of them are located about the periphery of the carbon ring, and are termed equatorial. The other six are oriented above and below the approximate plane of the ring (three in each location), and are termed axial because they are aligned parallel to the symmetry axis of the ring.
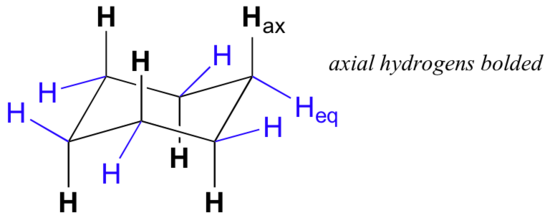
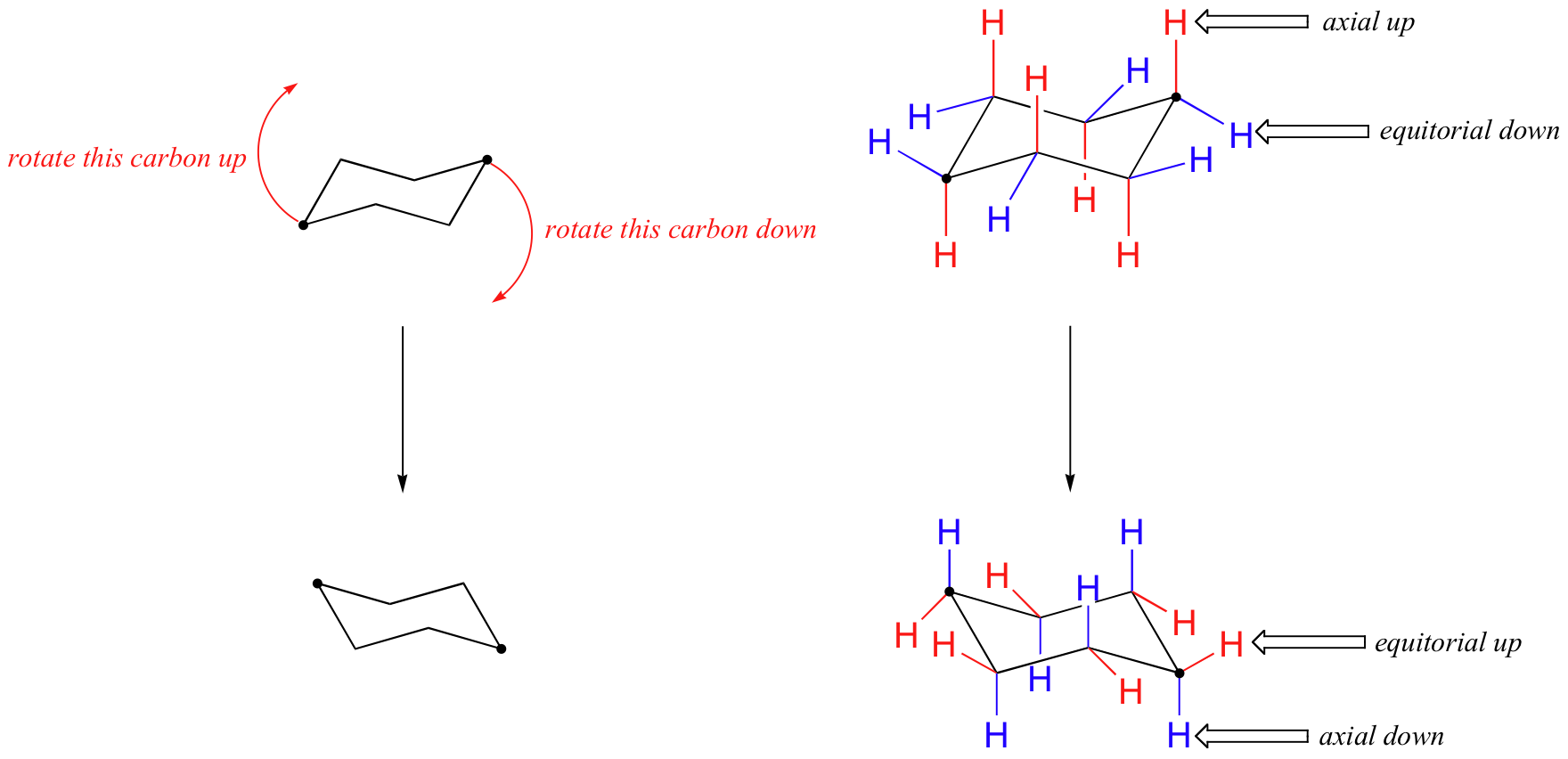
In the figure above, the equatorial hydrogens are colored blue, and the axial hydrogens are in bold. Since there are two equivalent chair conformations of cyclohexane in rapid equilibrium, all twelve hydrogens have 50% equatorial and 50% axial character. The figure below illustrates how to convert a molecular model of cyclohexane between two different chair conformations - this is something that you should practice with models. Notice that a 'ring flip' causes equatorial hydrogens to become axial, and vice-versa.
How to draw stereo bonds ("up" and "down" bonds)
There are various ways to show these orientations. The solid (dark) "up wedge" I used is certainly common. Some people use an analogous "down wedge", which is light, to indicate a down bond; unfortunately, there is no agreement as to which way the wedge should point, and you are left relying on the lightness of the wedge to know it is "down". The "down bond" avoids this wedge ambiguity, and just uses some kind of light line. The down bond I used (e.g., in Figure 5B) is a dashed line; IUPAC encourages a series of parallel lines, something like ![]() . What I did is a variation of what is recommended by IUPAC: http://www.chem.qmul.ac.uk/iupac/stereo/intro.html.
. What I did is a variation of what is recommended by IUPAC: http://www.chem.qmul.ac.uk/iupac/stereo/intro.html.
In ISIS/Draw, the "up wedge" and "down bond" that I used, along with other variations, are available from a tool button that may be labeled with any of them, depending on most recent use. It is located directly below the tool button for ordinary C-C bonds.
In Symyx Draw, the "up wedge" and "down bond", along with other variations, are available from a tool button that may be labeled with any of them, depending on most recent use. It is located directly below the "Chain" tool button.
ChemSketch provides up and down wedges, but not the simple up and down bonds discussed above. The wedges are available from the second toolbar across the top. For an expanded discussion of using these wedges, see the section of my ChemSketch Guide on Stereochemistry: Wedge bonds.
As always, the information provided on these pages in intended to help you get started. Each program has more options for drawing bonds than discussed here. When you feel the need, look around!
How to draw chairs
Most of the structures shown on this page were drawn with the free program ISIS/Draw. I have posted a guide to help you get started with ISIS/Draw. ISIS/Draw provides a simple cyclohexane (6-ring) hexagon template on the toolbar across the top. It provides templates for various 6-ring chair structures from the Templates menu; choose Rings. There are templates for simple chairs, without substituents (e.g., Fig 1B), and for chairs showing all the substituents (e.g., Fig 2B). In either case, you can add, delete, or change things as you wish. Various kinds of stereo bonds (wedges and bars) are available by clicking the left-side tool button that is just below the regular C-C single bond button. It may have a wedge shown on it, but this will vary depending on how it has been used. To choose a type of stereo bond, click on the button and hold the mouse click; a new menu will appear to the right of the button.
The free drawing program Symyx Draw, the successor to ISIS/Draw, provides similar templates and tools. A basic chair structure is provided on the default template bar that is shown. More options are available by choosing the Rings template. See my page Symyx Draw for a general guide for getting started with this program.
The free drawing program ChemSketch provides similar templates and tools. To find the special templates for chairs, go to the Templates menu, choose Template Window, and then choose "Rings" from the drop-down menu near upper left. See my page ChemSketch for a general guide for getting started with this program.
If you want to draw chair structures by hand (and if you are going on in organic chemistry, you should)... Be careful. The precise zigs and zags, and the angles of substituents are all important. Your textbook may offer you some hints for how to draw chairs. A short item in the Journal of Chemical Education offers a nice trick, showing how the chair can be thought of as consisting of an M and a W. The article is V Dragojlovic, A method for drawing the cyclohexane ring and its substituents. J Chem Educ 78:923, 7/01. (I thank M Farooq Wahab, Chemistry, Univ Karachi, for suggesting that this article be noted here.)
Aside from drawing the basic chair, the key points in adding substituents are:
- Axial groups alternate up and down, and are shown "vertical".
- Equatorial groups are approximately horizontal, but actually somewhat distorted from that, so that the angle from the axial group is a bit more than a right angle -- reflecting the common 109 degree bond angle.
- As cautioned before, it is usually easier to draw and see what is happening at the four corners of the chair than at the two middle positions. Try to use the corners as much as possible.
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

