1.6: The Divisible Atom
- Page ID
- 50989
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The opposite of a correct statement is a false statement. But the opposite of a profound truth may well be another profound truth. NEILS BOHR (1865–1962)
Dalton’s theory of atoms as indivisible, indestructible, objects of different sizes, weights, and perhaps shapes, depending on the element, held up for almost 100 years, although there was considerable dissent about whether atoms really existed, particularly among philosophers. By 1900 the atomic theory was almost universally accepted by chemists. More evidence began to accumulate, more elements were discovered, and it even became possible to calculate the number of atoms in a particular sample. The first step along this direction was made by Amedeo Avogadro (1776–1856). In 1811 he proposed that, under conditions of equal temperature and pressure, equal volumes of gases contained equal numbers of particles (molecules) and that the densities of the gases, that is their weight divided by their volume, were proportional to the weight of the individual molecules. This was expanded on by the Austrian high school teacher Josef Loschmidt (1821–1895) who, in 1865, combined Avogadro's conclusion with the assumption that atoms and molecules move very much as elastic objects, think billiard balls. This enabled him to calculate the force a molecule would exert when traveling at a particular speed, something difficult to measure, and relate that to the pressure, something easily measured. In fact, this assumption enabled physicists to deduce that the temperature of a gas is related to the average kinetic energy of the molecules within it, a concept we will return to shortly.
Probing the Substructure of Atoms
The initial Greek assumption was that atoms were indivisible, essentially unchangeable from their initial creation. However, gradually evidence began to accumulate that atoms were neither indivisible nor indestructible. Evidence for the existence of particles smaller than atoms had been building up for some time, although it was not recognized as such. For example, the well- recognized phenomenon of static electricity had been known since the ancient Greeks. The name electricity comes from the Latin electricus, meaning amber-like. Rubbing amber with fur generates static electricity—the same type of spark that jumps from your finger to a doorknob or another person under dry conditions. In the late 1700s Luigi Galvani (1737–1798) discovered that animals can produce and respond to electricity, perhaps the most dramatic example being the electric eels and rays that stun their prey through electrical shocks. The discovery of bioelectricity was exploited in many novels and movies, beginning with Mary Shelly's (1797–1851) novel Frankenstein and continuing through Mel Brook's (b. 1926) comedy film, Young Frankenstein. Galvani discovered that a dead frog’s leg would twitch in response to exposure to static electricity; it appeared to come back to life, just like Frankenstein’s monster. He assumed, correctly it turns out, that electrical activity was involved in the normal movement of animals. He thought that a specific form of electricity, bioelectricity, was carried in the fluid within the muscles and was a unique product of biological systems, a type of life-specific force. We now recognize that a number of biological phenomena, such as muscle contraction and brain activity, are initiated by changes in electric fields (across membranes) and that the underlying physicochemical principles are similar to those taking place in non-biological systems.
The excitement about electricity and its possible uses prompted Alessandro Volta (1745– 1827) to develop the first modern battery, now known as a voltaic pile. He alternated sheets of two different metals, such as zinc and copper, with discs soaked in salt water (brine). It produced the first steady electrical current that, when applied to frog muscles, caused them to contract. Such observations indicated that biological systems can both generate and respond to electrical currents, suggesting that bioelectricity was no different than any other form of electricity. What neither Volta nor Galvani knew was the nature of electricity. What was it, exactly, and how did it flow from place to place? What was in the spark that jumped from finger to metal doorknob, or from Benjamin Franklin's (1705–1790) kite string to his finger? What was this “electrical fluid” made of?
Progress in the understanding of the nature and behavior of electricity continued throughout the 19th century and the power of electricity was harnessed to produce dramatic changes in the way people lived and worked, powering factories, lighting houses and streets, and so on. Yet there was no deep of understanding as to the physical nature of electricity. It was known that electric charge came in two forms, positive and negative, and that these charges were conserved; that is, they could not be created or destroyed, ideas first proposed by Franklin. The electrical (charged) nature of matter was well established, but not where those charges came from or what they were.
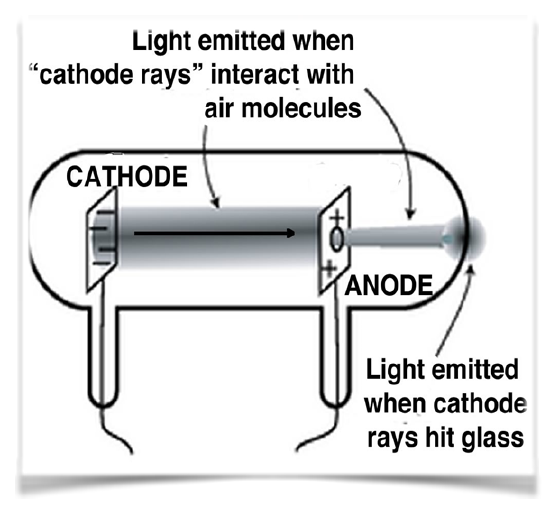
A key step to understanding electricity involved unravelling the idea of the indivisible atom and involved a series of experiments by J. J. Thompson (1856–1940), another Mancunian.16 Although the idea of electricity was now well appreciated, Thompson and other scientists wanted to study it in a more controlled manner. They used what were, and are now, known as cathode ray tubes (CRTs) shown in Figure 1.6.1. Once common in televisions, these have now been replaced by various flat screen devices. CRTs are glass tubes with wires embedded in them; these wires are connected to metal discs. The inside of the tube is coated with a chemical that glows (fluoresces) in response to electricity. They generally have ports in the walls that can be connected to a vacuum pump, so that most of the air within the tube can be removed, typically the ports are then sealed. When connected to a source of electricity, such as a voltaic pile, the fluorescent material at one end of the tube glows. In a series of experiments (1897) Thompson was able to show that:
- Rays emerged from one disc (the cathode) and moved to the other (the anode).
- These “cathode” rays were deflected by electrical fields in a direction that indicated that they were negatively charged.
- The rays could also be deflected by magnetic fields.17
- The rays carried the electrical charge; that is, if the ray was bent, for example by a magnetic field, the charge went with it.
- The metal that the cathode was made of did not affect the behavior of the ray; so whatever the composition of the ray, it appeared to be independent of the element that it came from.
In all of these experiments, it needs to be stressed that "positive" and "negative" are meant to indicate opposite and are assigned by convention. That means that we could decide tomorrow that positive was negative, and negative positive, and nothing would change, as long as we were consistent. From these experiments, Thompson concluded that “cathode” rays were carried by discrete charged particles, he called them corpuscles, and he assigned these particles a negative charge. But the truly stunning conclusion he reached was that these particles must come from within the atoms of the metal cathode. Because the type of metal did not affect the nature or behavior of the cathode rays, he assumed that these particles were not newly created but must pre-exist within the atoms of the cathode. Moreover, he hypothesized that identical particles must be present in all atoms, not just in the atoms of one particular metal. Do you see how he jumps from experimental results using a few metals to all elements and all atoms? Of course, we now know these particles as electrons but it is difficult to imagine what a huge impact this new theory had on scientists at the time.
Since electrons can be produced by all chemical elements, we must conclude that they enter the constitution of all atoms. We have thus taken our first step in understanding the structure of the atom. —J. J. Thompson, The Atomic Theory, 191418
The discovery of the electron made the old idea of an atom as a little indestructible billiard ball-like objects obsolete, and necessitated a new model. It is an example of a paradigm shift19—a fundamental change in scientific thinking driven by new evidence. Thompson’s first version of this new model became known as the plum pudding model.20 His basic idea was that the atom is a ball of positively charged, but apparently amorphous, matter with electrons studded here and there, like the raisins in a pudding. Because it contained equal numbers of positive and negative charges, the overall structure was electrically neutral. Subsequent work by Thompson and Robert A. Millikan (1868–1953) established that all electrons are identical, each with the same, very small mass and negative charge. The mass of an electron is less than 1/1000th of the mass of a hydrogen atom.
Thompson's proposed plum pudding model of the atom spurred much experimental and theoretical work and led to a remarkable number of subsequent discoveries. For example, it was soon recognized that the β particles emitted by some radioactive minerals and elements, were, in fact, electrons. Other studies found that the number of electrons present in the atoms of a particular element was roughly proportional to half the element's atomic weight, although why this should be the case was unclear.
However, as more and more data began to accumulate, the plum pudding model had to be abandoned because it just could not explain what was being observed. The key experiment that led to a new model of the atom was carried out in 1908 by Ernest Rutherford (1871–1937). As you may have already guessed, he was working at the University of Manchester. In this experiment, he examined how alpha (α) particles, which he knew to be positively charged particles made of the element helium without it’s electrons, behaved when they were fired at a very thin sheet of metal, such as gold or platinum. In the experiment a narrow parallel beam of α particles was directed at a thin sheet of gold foil and the angles at which the deflected particles scattered were detected. The observed result was completely unexpected. Instead of passing straight through the thin sheet of foil, he found that a few particles were deflected, some of them at large angles. Rutherford wrote, “It is as if I had fired a cannon ball at a piece of tissue paper, and it bounced right back.” Here again, we see a particular aspect of the scientific enterprise, namely that even though only a few alpha particles bounced back, we still need to explain how this could possibly occur. We could not just say, "Only a few particles were bounced so it doesn't matter"; we have to provide a plausible scenario to explain the observation. Often it is paying attention to, and taking seriously, the unexpected result that leads to the most profound discoveries.
Based on these experimental results Rutherford reasoned that the positively charged α particles were being repelled by positive parts of the atom. Because only a very small percentage of alpha particles were deflected, only a very small region of each atom could be positively charged. That is, the positive charge in an atom could not be spread out more or less uniformly, as the plum pudding model assumed; instead it must be concentrated in a very small region. This implied that most of the atom is empty (remember the void of the ancient Greeks?) or occupied by something that poses little or no resistance to the passage of the α particles. What it left unexplained was why positively charged particles (which we now know as protons) concentrated in such a small volume, did not repel one another – the answer to which had to wait to discovery of the strong nuclear force (see below). Again we see a scientist making a huge intuitive leap from the experimental observation to a hypothesis that was consistent with that evidence and that makes specific predictions that can be confirmed or falsified by further experiment and observation. Rutherford's model, which became known as the planetary model, postulated a very, very small nucleus where all of the positive charge and nearly all of the mass of the atom was located; this nucleus was encircled by electrons. In 1920 Rutherford went on to identify the unit of positive charge and called it the proton. In 1932 James Chadwick (1891–1974)(who co- incidentally studied at the University of Manchester) identified a second component of the nucleus, the neutron. Neutrons are heavy, like protons. In fact they are slightly heavier than protons, but have no charge. The identity of the element depends on the number of protons, however the number of neutrons may be different in different atoms of the same element. For example an atom of carbon always has six protons, but it can have different numbers of neutrons. Most carbon atoms have six neutrons (C-12), but some have seven (C-13) and some have eight (C-14).
Questions for Later
- If atoms are mostly empty space, why can’t we walk through walls?
- What is radiation?
- How does an atom change when it emits an alpha particle? Or a beta particle/electron?
Questions to Ponder
- If the original discoverers of electricity had decided that electrons have a positive charge, would that have made a difference in our understanding of electricity?
- Why do you think electrons were the first sub-atomic particles to be discovered?
- How exactly did Rutherford detect alpha particles?
- Can you think of an alternative model of the atom based on Rutherford's observations?
- How would the experiment change if he had used electrons or neutrons?
References
16 That is, a person from Manchester, England.
17 This works because the electrons are spinning.
18 http://www.aip.org/history/electron/jjsound.htm
19 A term made popular (although often misunderstood) by T. S. Kuhn, The Structure of Scientific Revolutions, 1st. ed., Chicago: Univ. of Chicago Pr., 1962
20 This can be a little confusing to those not familiar with plum pudding – a “delicious” English delicacy composed of dried fruit (raisins) in a spongy base, usually prepared by boiling for several days and often served with rum sauce.
Contributors
M.M. Cooper (Michigan State University) and Mike W. Klymkowsky (University of Colorado, Boulder)

