Theoretical Background (teachers)
- Page ID
- 60665
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electromagnetic radiation
Electromagnetic radiation (EMR) is the type of energy that encompasses light, heat, and x-rays. It can be conveniently described using a sinusoidal wave model, where the properties of the radiation depend on the wavelength, frequency, and other parameters of the wave. For some purposes, usually when discussing the absorption and transmittance of the energy of the radiation, it makes more sense to describe the energy as a stream of light particles called photons, where the energy of the photons is proportional to the frequency of the radiation. The wave/particle duality applies to all elementary particles, and should be used as a complementary, rather than contradictory, description of the movement of the radiation.
Wave properties of electromagnetic radiation
Some useful definitions and equations:
- Amplitude (A): The height of the wave (fig. 1)
- Wavelength (λ) : The distance between two crests of the wave (fig. 1)
- Crest and trough : The highest and lowest points, respectively, of a wave (fig. 1)
- Speed of light ( c ) - The velocity of radiation as it travels through a vacuum. This quantity is the same for all forms of electromagnetic radiation, from x-rays to light to radio waves, and is constant within a particular transportation medium.The speed of light in vacuum is 2.99792 x 108 m/s. The speed of light in air is only 0.03% slower, and c in either medium is usually just rounded off to 3.00 x 108 m/s.
- Frequency (ν) : The number of waves that pass a fixed point per second
- Period (T) : The number of seconds it takes for a wave to pass a fixed point
Figure 1: The features of a sinusoidal wave.
- ν = 1/T - The frequency of the wave is the reciprocal of the period.
- λ ν = c (or ν = c/λ) - the product of frequency and wavelength is the speed of light. Alternatively, the frequency of a wave is inversely proportional to the velocity.
- E = hν = hc/λ , where h is the Planck constant, 6.626 x 10-34 - The energy of the radiation is equal to the Plank constant multiplied by the frequency of the radiation.
The Electromagnetic Spectrum
Although all waves of electromagnetic radiation travel at the speed of light, different types of waves have vastly differing wavelengths, frequencies, and energies. The shorter the wavelength of the radiation, the greater the frequency and the larger the energy. The electromagnetic spectrum ranges from gamma (γ) radiation, which has the shortest wavelength, highest frequency, and greatest energy, to radio waves, which has the longest wavelength and lowest frequency and energy.
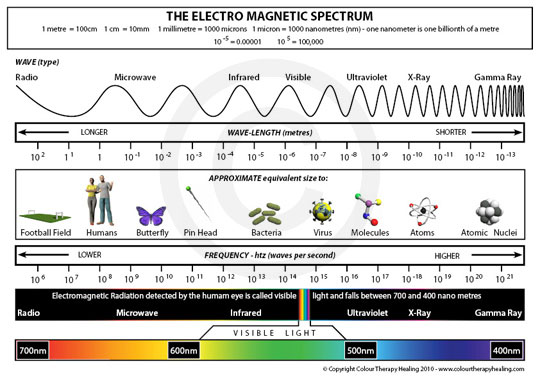
Figure 2: The electromagnetic spectrum, showing relative wavelengths and frequencies, with visible light expanded.
Movement of light between two substances
When light moves between two substances, both the speed and the direction of the electromagnetic wave will change. The refractive index, n, is given by the equation
n=c/vp
where c is the speed of light in vacuum and vp is the speed of light in that particular substance. The refractive index is unitless and simply provides a means to compare the relative speeds of light in transparent substances; the larger the refractive index of a material, the slower light will pass through it.
When the wave of light changes velocity, it also changes direction (figure 3). This refraction can be related to the speeds of light in the substances using Snell's Law,
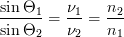
where θ1 is the angle of incidence, θ2 is the angle of reflection, and v1 and v2 are the speeds of light in the first and second media, respectively.
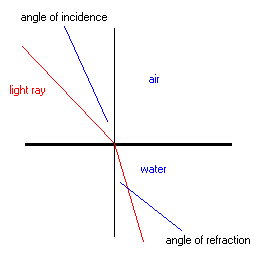
Fig. 3: Refraction of light: a change in the direction of a wave due to a change in speed
In vacuum all types of radiation are refracted equally, but in other media the refractive index is related to the wavelength of the light. Because of this, light of different wavelengths is refracted to different degrees. This phenomenon is known as dispersion.
Dispersion of White Light in Gratings
White light is a mixture of all of the wavelengths of visible light. When a beam of white light is sent from one medium to another, the different wavelengths of light that make up the beam are refracted at different angles because they are traveling at different speeds and have different refractive indexes. This causes the different wavelengths to be separated from each other into the visible spectrum as shown in Figure 4.
Light can be separated using either prisms (Figure 4) or diffraction gratings (Figure 5).
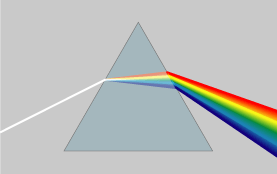
Figure 4: A beam of white light dispersing into its component colors.
A transmission diffraction grating is made up of a transparent material with regularly spaced grooves cut into it so that a beam of light passing through is separated out into the component wavelengths.
Figure 5: Dispersion of light in a transmission grating
The separation of the light is governed by the equation:
nλ = d(sin i + sin r)
where n is the diffraction order (a small whole number), d is the spacing between the grooves of the grating (usually calculated in nanometers/groove), and i and r are the angles of incidence and refraction, respectively.
Because there are several values for 'n', there are a number of spectra, found at different angles of refraction, which can be formed from a diffraction grating. Ordinarily, however, the first-order line is the most intense, and only the +1, 0, and -1 spectra can be seen.
Spectrophotometers
Spectrophotometers are instruments that measure the absorbance of wavelengths of light in solutions. The absorbance, A of a solution is a measure of how much light of a certain wavelength specific to the experiment passes through a solution versus how much is absorbed by the solution. Absorbance is defined using the Beer's Law
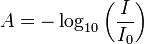
where I0 is the amount of the experimental wavelength of light present before the beam of light passes through the solution, and I is the amount of light present in the beam after it has passed through the solution. In general, the darker the solution, the less light that passes through the solution and the higher the absorbance.
Using our spectrophotometer, the students will be able to see which wavelengths of light in the visible spectrum are absorbed and which are transmitted. A deep red transparent solution, for example, allows red and orange light to pass through and absorbs the other colors in the spectrum. A pale red solution absorbs some of the blue and green light, but also lets some through, and a clear solution transmits the entire visible spectrum. This can be clearly seen in the diffracted spectrum through the grating.


