6.11 E2 Elimination
- Page ID
- 15776
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
E2 reactions are typically seen with secondary and tertiary alkyl halides, but a hindered base is necessary with a primary halide. The mechanism by which it occurs is a single step concerted reaction with one transition state. The rate at which this mechanism occurs is second order kinetics, and depends on both the base and alkyl halide. A good leaving group is required because it is involved in the rate determining step. The leaving groups must be coplanar in order to form a pi bond; carbons go from sp3 to sp2 hybridization states.
General Reaction
In this reaction Ba represents the base and X represents a leaving group, typically a halogen. There is one transition state that shows the concerted reaction for the base attracting the hydrogen and the halogen taking the electrons from the bond. The product be both eclipse and staggered depending on the transition states. Eclipsed products have a synperiplanar transition states, while staggered products have antiperiplanar transition states. Staggered conformation is usually the major product because of its lower energy confirmation.
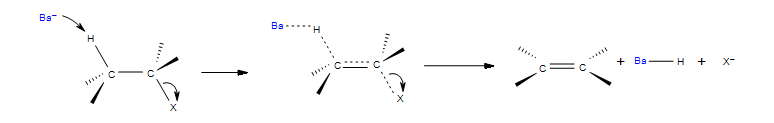
An E2 reaction has certain requirements to proceed:
- Secondary and tertiary alkyl halides will proceed with E2 in the presence of a base (OH-, RO-, R2N-)
- Both leaving groups should be on the same plane, this allows the double bond to form in the reaction. In the reaction above you can see both leaving groups are in the plane of the carbons.
- Follows Zaitsev's rule, the most substituted alkene is usually the major product.
- Hoffman Rule, if a sterically hindered base will result in the least substituted product.
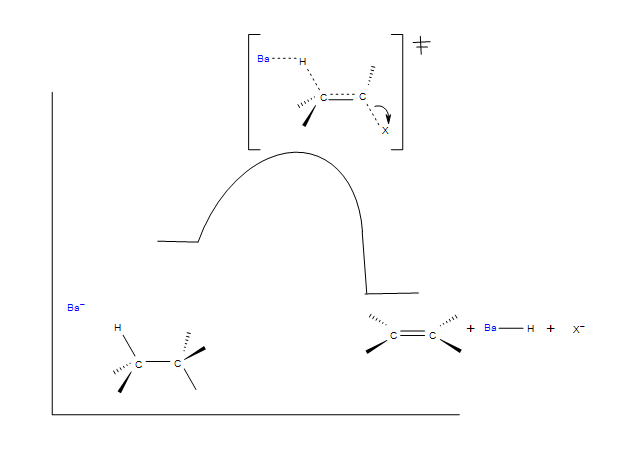
Reaction Coordinate
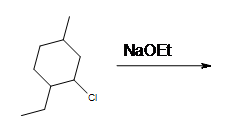
Problems
1.
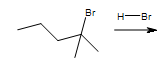
2. What is the major product and why?
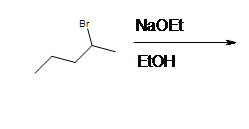
3. What is the major prodcut when 2-bromo-2-methylbutane reacts with with sodium ethoxide?
4.
5.
Further Reading
Michigan State Virtual Textbook of Organic Chemistry
MasterOrganicChemistry
Elimination Reactions And Cyclohexane Rings
Carey 4th Edition On-Line Activity
Khan Academy
Leah4Sci
E2 Reaction Rate & Mechanism (vid 1 of 4) Bimolecular Beta-Elimination by Leah Fisch
E2 Reaction (vid 2 of 4) Using Newman Projections To Predict Products by Leah4sci
E2 reaction (vid 3 of 4) using chair conformations for anti coplanar reactions
E2 Reaction (vid 4 of 4) Big Bulky Base for Anti-Zaitsev Product
Cliffs Notes
Slide Presentations
Web Pages
Videos

