3.6: Steady State Approximation
- Page ID
- 92297
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The steady state approximation is a method used to estimate the overall reaction rate of a multi-step reaction. It assumes that the rate of change of intermediate concentration in a multi-step reaction are constant. This method can only be applied when the first step of the reaction is significantly slower than subsequent step in an intermediate-forming consecutive reaction.
Introduction
Before discussing the steady state approximation, it must be understood that the approximation is derived to simplify the kinetic expression for product concentration, [product]. Consider the following sequential reaction:
\[A \xrightarrow[]{k_1} B \xrightarrow {k_2} C\]
Calculating the [product] depends on all the rate constants in each step. For example, if the kinetic method was used to find the concentration of C, [C], at time t in the above reaction, the expression would be
\[[C] = [A]_0 \left(1+ \dfrac{k_2e^{-k_1t}-k_1e^{-k_2t})}{k_1 - k_2}\right) \label{1}\]
With a more complicated mechanisms, the kinetic expression becomes harder to derive. To simplify this calculation, scientists developed the steady state approximation and the pre-equilibrium approximation for determining the overall reaction rates of consecutive reactions. This article concerns the steady state approximation.
Steady State Approximation
The steady state approximation is applies to a consecutive reaction with a slow first step and a fast second step (\(k_1<<k_2\)). If the first step is very slow in comparison to the second step, there is no accumulation of intermediate product, such as product B in the above example.
\[\dfrac{d[B]}{dt} = 0 = k_1[A] - k_2[B] \label{2}\]
Thus
\[[B] = \dfrac{k_1[A]}{k_2} \label{3}\]
From the mechanism:
\[\dfrac{d[C]}{dt} = k_2[B] = \dfrac{k_2k_1[A]}{k_2} = k_1[A] \label{4}\]
Solving for \([C]\):
\[[C] = [A]_0 (1- e^{-k_1t}) \label{5}\]
Equation \(\ref{2}\) is much simpler to derive than Equation \(\ref{1}\) , especially with a more complicated reaction mechanisms.
Example \(\PageIndex{1}\)
Consider the reaction:
\(A + 2B \xrightarrow[]{} C\)
A: What is the expected rate law according to the following proposed multi-step mechanism under the steady state approximation with \(k_2 >> k_{-1}\)) for the following mechanism:
- \(A + B \xrightarrow[k_{-1}]{k_1} I\) Slow
- \(I + B \xrightarrow[]{k_2} C\) Fast
A:
\[\dfrac{d[I]}{dt} = k_1[A][B] - k_{-1}[I] - k_2[I][B] = 0\]
\[[I] = \dfrac{k_1[A][B]}{ k_{-1} +k_2[B]}\]
because \(k_2>>k_{-1}\) then \(k_{-1} = 0\). Therefore, \([I] = \dfrac{k_1[A]}{k_2}\)
\[\dfrac{d[C]}{dt} = k_2[I][B]\]
\[\dfrac{d[C]}{dt} = \dfrac{k_1k_2[A][B]}{k_2}\]
\[\dfrac{d[C]}{dt} = k_1[A][B]\]
B:
- \(x = 1\)
- \(y = 1\)
- \(n = 2\)
Use of the Steady-State Approximation in Enzyme Kinetics
In 1925, George E. Briggs and John B. S. Haldane applied the steady state approximation method to determine the rate law of the enzyme-catalyzed reaction (Figure 1). The following assumptions were made:
- The rate constant of the first step must be slower than the rate constant of the second step (\(k_1 << k_2\)), hence \[\dfrac{d[ES]}{dt} = 0\]
- Enzyme concentration must be significantly lower than the substrate concentration to keep the first step slower than the second step.
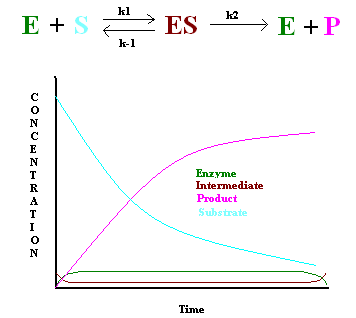
Figure 1: Steady state dynamicsin enzymes
This gives the following:
\[\dfrac{d[P]}{dt} = k_2[ES] \label{6}\]
where
\[\dfrac{d[ES]}{dt} = 0 = k_1[E][S] - k_{-1}[ES] - k_2[ES] \label{7}\]
Because
\[[S] >> [E] \label{8}\]
Using the second assumption and the fact that enzyme concentration equals the initial concentration of enzyme minus the concentration of the enzyme-substrate intermediate,
\[[E] = [E]_o - [ES] \label{9}\]
The following equation is obtained:
\[k_1[E]_o[S] = k_{-1}[ES] + k_2[ES] + k_1[ES][S] \label{10}\]
From this equation, the concentration of the ES intermediate can be found:
\[[ES] = \dfrac{k_1[E]_o[S]}{(k_{-1} + k_2) + k_1[S]} \label{11}\]
Substitute this into Equation \(\ref{3}\) gives,
\[\dfrac{d[P]}{dt} = \dfrac{k_2[E]_0[S]}{[(k_1+k_2)/k_1]+[S]} = \dfrac{k_2[E]_0[S]}{(K_M+[S]} \label{12}\]
where
\[K_M = \dfrac{k_{-1}+k_{2}}{k_1} \label{13}\]
Because in most of the cases, only the initial d[P]/dt is measured to determine the rate of product formation, (4) can be rewritten as:
\[v_0 = \dfrac{d[P]_0}{dt} = \dfrac{k_2[E]_0[S]}{K_M+[S]} \label{14}\]
Because [E]0 = Vmax/k2. Equation \(\ref{5}\) becomes the following:
\[v_0 = \dfrac{d[P]_0}{dt} = \dfrac{k_2/k_2)v_{max}[S]}{(K_M+[S]} \label{15}\]
\[\\ = \dfrac{V_{max}[S]}{K_M+[S]} \label{16}\]
This equation is a very useful tool to in calculating vmax and KM (the Michaelis constant), of an enzyme by using the Lineweaver-Burk plot (1/[S] vs. 1/v0) or the Eadie-Hofstee plot (v0/[S] vs. v0).
Problems
Given the reaction \(A \xrightarrow[]{k_1} B \xrightarrow[]{k_2} C\)
where k1= 0.2 M-1s-1 , k2 = 2000 s-1
- Write the reaction rates for A, B, and C.
- Is this a steady-state reaction?
- Write the expression for d[C]/dt using the Steady State Approximation
- Calculate d[C]/dt if [A] = 1M
- Calculate [C] at t = 3 s and [A]0 = 2M
Solutions
1) d[A]/dt = -k1[A]; d[B]/dt = k1[A] - k2[B]; d[C]/dt = k2[B]
2) Because k1 is much larger than k2, this is a steady state reaction.
3) d[C]/dt = k2[B]
where d[B]/dt = k1[A] - k2[B] = 0
so, [B] = k1[A]/k2
Substitute this into d[C]/dt
d[C]/dt = k1[A]
4) d[C]/dt = 0.2M-1s-1(1M) = 0.2 s-1
5) [C] = [A]0 (1-e-k1t) = 2M(1-e-0.2(3)) = 0.9 M
References:
- Chang, Raymond. Physical Chemistry for The Biosciences. Sausalito: University Science Books, 2005. 368-370.
- Garrett, Reginald H, Charles M. Grisham. Biochemistry. 4th ed. Boston: Brooks/Cole Cengage Learning, 2010. 389-397.
- Segel, Irwin H. Biochemical Calculations. 2nd ed. New Jersey: John Wiley and Sons, inc., 1976. 216-218.
Contributors
- Melanie Miner, Tu Quach, Eva Tan, Michael Cheung

