Answers to Chapter 10 Study Questions
- Page ID
- 11311
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
-
- \(\ce{NH3}\) (\(\textrm{H-bonds}\))/\(\ce{PH3}\) (dipole); \(\ce{NH3}\)
- both LDF; \(\ce{C6H14}\) (higher molar mass)
- \(\ce{CO2}\) (LDF)/\(\ce{H2O}\) (\(\textrm{H-bonds}\); \(\ce{H2O}\)) (also, \(\ce{H2O}\) is a liquid, while \(\ce{CO2}\) is a gas)
- \(\ce{HCl}\) (dipole)/\(\ce{LiCl}\) (ionic); \(\ce{LiCl}\)
- \(\ce{Na}\) (metallic)/\(\ce{NaCl}\) (ionic); \(\ce{NaCl}\)
-
- Ionic solids are composed of positive and negative ions arranged so that each positive ion is surrounded by negative ions and vice versa. The are held together by the attractive force between the oppositely charged ions. Some examples are \(\ce{NaCl}\), \(\ce{NaNO3}\), \(\ce{MgSO4}\), \(\ce{CuCl2}\).
- Covalent (molecular) solids are made up of molecules. Covalent bonds hold together the atoms within the molecules. The forces between the molecules are dispersion and dipole forces and hydrogen bonds. Examples include water (\(\ce{H2O}\)), ammonia (\(\ce{NH3}\)), table sugar (\(\ce{C12H22O11}\)), \(\ce{CO2}\).
- Metallic solids are made up of a regular crystalline array of metal atoms. Valence electrons are free to move within the solid and make up the "metallic bonding" that holds the metal together. Some metallic solids are aluminum, gold, iron, nickel, copper, zinc, sodium.
- Network covalent solids consist of atoms covalently bound together in a continuous 3-dimensional network. The solid is held together by covalent bonds. Examples are diamond (\(\ce{C}\)), carborundum (\(\ce{SiC}\)) and quartz (\(\ce{SiO2}\)).
- In general, b) molecular < c) metallic < a) ionic < d) covalent network
- Only metallic substances conduct electricity as solids. Both metallic and ionic substances conduct electricity as liquids.
-
- \(\ce{F2}\) (molecular, LDF)
- \(\ce{NaCl}\)
- The boiling point of a liquid is the temperature at which its equilibrium vapor pressure is equal to external pressure. The critical temperature is the highest temperature a substance can be liquified; above the critical temperature a substance is a gas at any pressure. The critical pressure is the pressure needed to liquify a substance at its critical temperature. The triple point is the temperature and pressure at which solid, liquid and gas all exist in equilibrium.
-
- no change
- vapor pressure increases with increasing temperature
- higher intermolecular forces mean lower vapor pressure
- no change
- London dispersion forces (van der Waals forces) < dipole-dipole forces < hydrogen bonds.
-
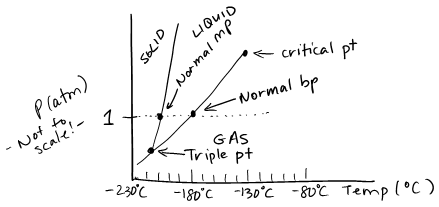
- \(\ce{O2 (s)}\) is denser than \(\ce{O2 (l)}\) because the solid-liquid line on the phase diagram is normal (slants slightly to the right).
- \(\ce{O2}\) will melt when heated at a pressure of 1 atm.
- First find the number of moles of iodine in the flask, using the ideal gas law:
\(\mathrm{P = 0.466\: mmHg = 6.13 \times 10^{-4}\: atm}\); \(\mathrm{T = 30^\circ C = 303\: K}\); \(\mathrm{V = 1.00\: L}\); \(\mathrm{n = ?}\)
\(\mathrm{n=\dfrac{PV}{RT}=\dfrac{(6.13\times10^{-4}\:atm)(1.00\:L)}{(0.0821)(303\:K)}=2.46 \times 10^{-5}\: moles}\)
\(\mathrm{2.46\times10^{-5}\:moles\times\dfrac{253.8\:g\:I_2}{1\:mole\:I_2}\times\dfrac{1000\:mg}{1\:g}=6.25\:mg}\)


