21.16: 21.8 Chemistry of Acid Halides
- Page ID
- 144071
Objectives
After completing this section, you should be able to
- identify the reagent normally used to convert a carboxylic acid to an acid bromide.
- write equations to show how an acid halide may be converted into each of the following: a carboxylic acid, an ester, an amide.
- write a detailed mechanisms for the reaction of an acid halide with each of the following: water, an alcohol, ammonia, a primary or secondary amine.
- identify the product formed when a given acid halide reacts with any of the following reagents: water, an alcohol, a primary or secondary amine.
- identify the acid halide, the reagents, or both, needed to prepare a given carboxylic acid, ester or amide.
- identify the product formed when a given acid halide reacts with water, a given alcohol, ammonia, or a given primary or secondary amine.
- identify lithium aluminum hydride as a reagent for reducing acid halides to primary alcohols, and explain the limited practical value of this reaction.
- identify the partial reduction of an acid halide using lithium tri‑tert‑butoxyaluminum to form an aldehyde.
- write an equation to describe the formation of a tertiary alcohol by the reaction of an acid halide with a Grignard reagent.
- write a detailed mechanism for the reaction of an acid halide with a Grignard reagent.
- identify the product formed from the reaction of a given acid halide with a given Grignard reagent.
- identify the acid halide, the Grignard reagent, or both, needed to prepare a given tertiary alcohol.
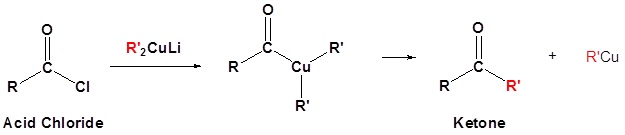
- write an equation to illustrate the reaction of an acid halide with a lithium diorganocopper reagent.
- identify the product formed from the reaction of a given acid halide with a given lithium diorganocopper reagent.
- identify the acid halide, the lithium diorganocopper reagent, or both, that must be used to prepare a given ketone.
Study Notes
This figure provides a convenient general summary of a few of the reactions described in Section 21.4.
Note that LiAlH4 is a common reagent for hydride [H−] reduction of and acid chloride to an alcohol.
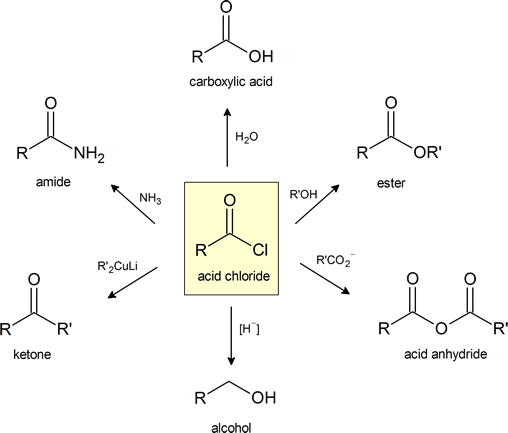
Formation of Acid Halides
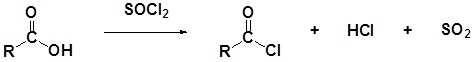
Carboxylic acids react with thionyl chloride (\(SOCl_2\)) to form acid chlorides. During the reaction the hydroxyl group of the carboxylic acid is converted to a chlorosulfite intermediate making it a better leaving group. The chloride anion produced during the reaction acts a nucleophile.
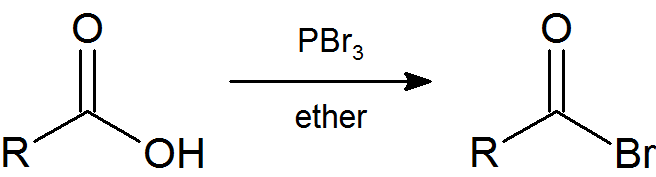
Earlier (Section 10.5) we saw that primary and secondary alcohols react with PBr3 to afford the corresponding alkyl bromide. In a similar fashion acid bromides can be formed from the carboxylic acid.
Nucleophilic Acyl Substitution Mechanism
If you understand the mechanism of a typical nucleophilic acyl substitution, the reaction of an acyl halide with water, an alcohol or ammonia should not present you with any difficulty.
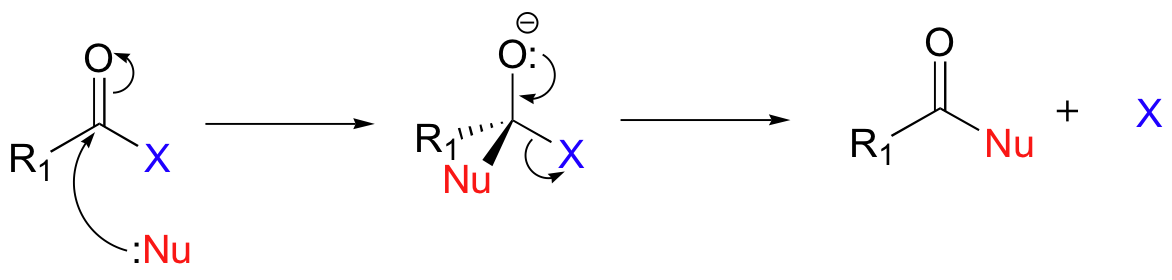
X = Cl, Br :Nu = H2O, ROH, NH2R, NHR2 etc.
Acid chlorides react with water to form carboxylic acids.
General reaction
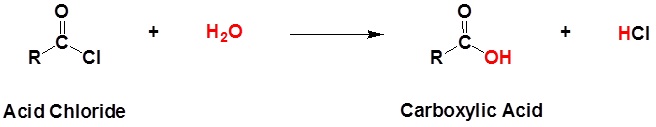
Example 21.4.1

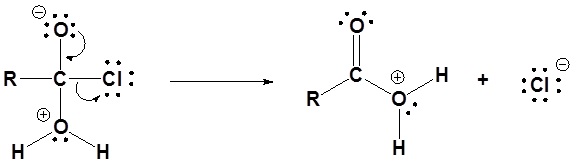
Mechanism
1) Nucleophilic attack by water

2) Leaving group is removed
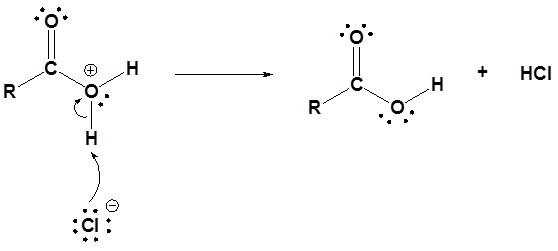
3) Deprotonation
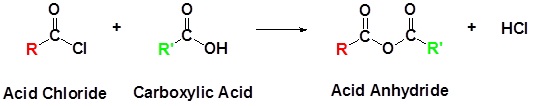
Acid chlorides react with carboxylic acids to form anhydrides
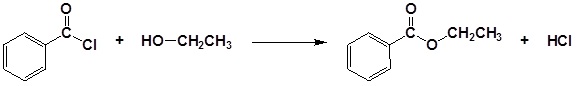
Acid chlorides react with alcohols to form esters
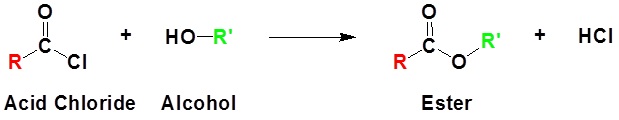
Example 21.4.2
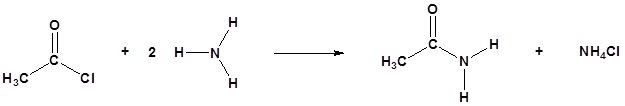
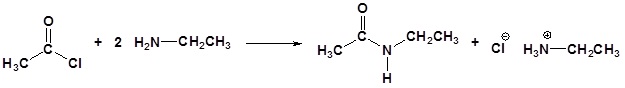
Acid chlorides react with ammonia, 1o amines and 2o amines to form amides.
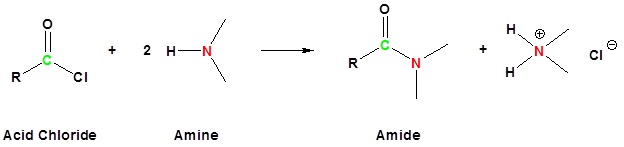
Example 21.4.3
Acid Chlorides can be Reduced to form 1o Alcohols
Grignard reagents convert acid chloride to 3o alcohols
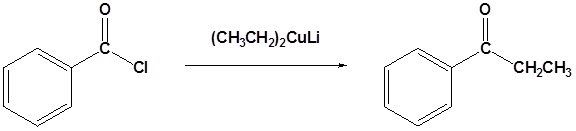
Organocuprate reagents convert acid chlorides to ketones.
Example 21.4.4
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

