13.13: Oxidation Reactions of Alcohols
- Page ID
- 131391
Objectives
After completing this section, you should be able to
- write an equation to represent the oxidation of an alcohol.
- identify the reagents that may be used to oxidize a given alcohol.
- identify the specific reagent that is used to oxidize primary alcohols to aldehydes rather than to carboxylic acids.
- identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent.
- identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple oxidation.
- write a mechanism for the oxidation of an alcohol using a chromium(VI) reagent.
Study Notes
The reading mentions that pyridinium chlorochromate (PCC) is a milder version of chromic acid that is suitable for converting a primary alcohol into an aldehyde without oxidizing it all the way to a carboxylic acid. This reagent is being replaced in laboratories by Dess‑Martin periodinane (DMP), which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous reaction conditions. DMP is named after Daniel Dess and James Martin, who developed it in 1983.
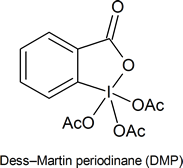
Oxidizing the different types of alcohols
The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromate(VI) acidified with dilute sulfuric acid. If oxidation occurs, the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions. The electron-half-equation for this reaction is
\[ Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O \tag{17.7.1}\]
Oxidizing agents
- K2Cr2O7 potassium dichromate
- CrO3 Chromium Trioxide
Both of these are used along with H2SO4, H2O
- 1o alcohol → Carboxylic acid
- 2o alcohol → Ketone
- 3o alcohol → No reaction
Primary alcohols
Primary alcohols can be oxidized to either aldehydes or carboxylic acids depending on the reaction conditions. In the case of the formation of carboxylic acids, the alcohol is first oxidized to an aldehyde which is then oxidized further to the acid.
Full oxidation to carboxylic acids
You need to use an excess of the oxidizing agent and make sure that the aldehyde formed as the half-way product stays in the mixture. The alcohol is heated under reflux with an excess of the oxidizing agent. When the reaction is complete, the carboxylic acid is distilled off. The full equation for the oxidation of ethanol to ethanoic acid is:
\[ 3CH_3CH_2OH + 2Cr_2O_7^{2-} + 16H+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O \tag{17.7.1}\]
The more usual simplified version looks like this:
\[ CH_3CH_2OH + 2[O] \rightarrow CH_3COOH + H_2O \tag{17.7.2}\]
Alternatively, you could write separate equations for the two stages of the reaction - the formation of ethanal and then its subsequent oxidation.
\[ CH_3CH_2OH + [O] \rightarrow CH_3CHO + H_2O \tag{17.7.3}\]
\[ CH_3CHO + [O] \rightarrow CH_3COOH \tag{17.7.4}\]
This is what is happening in the second stage:

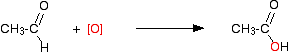
Secondary alcohols
Secondary alcohols are oxidized to ketones - and that's it. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate(VI) solution acidified with dilute sulfuric acid, you get propanone formed. Playing around with the reaction conditions makes no difference whatsoever to the product. Using the simple version of the equation and showing the relationship between the structures:

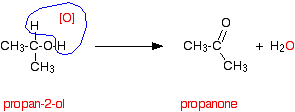
If you look back at the second stage of the primary alcohol reaction, you will see that an oxygen "slotted in" between the carbon and the hydrogen in the aldehyde group to produce the carboxylic acid. In this case, there is no such hydrogen - and the reaction has nowhere further to go.
Tertiary alcohols
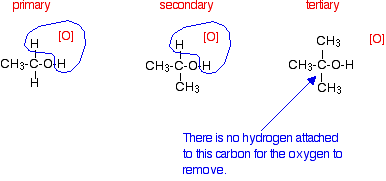
Tertiary alcohols are not oxidized by acidified sodium or potassium dichromate(VI) solution - there is no reaction whatsoever. If you look at what is happening with primary and secondary alcohols, you will see that the oxidizing agent is removing the hydrogen from the -OH group, and a hydrogen from the carbon atom attached to the -OH. Tertiary alcohols don't have a hydrogen atom attached to that carbon.
You need to be able to remove those two particular hydrogen atoms in order to set up the carbon-oxygen double bond.
Mechanism
Examples
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)
James Ashenhurst (MasterOrganicChemistry.com)

