9.3: Aromatic Compounds- Benzene and Its Relatives
- Page ID
- 483454
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Name and identify aromatic compounds.
Benzene is the parent compound of the large family of organic compounds known as aromatic compounds. Unlike cyclohexane, benzene only contains six hydrogen atoms, giving the impression that the ring is unsaturated and each carbon atom participates in one double bond. Two different structures with alternating single and double bonds around the ring can be written for benzene.
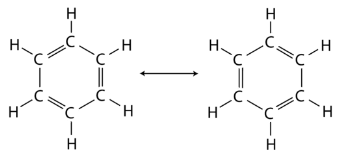
In benzene, the true bonding between carbon atoms is neither a single nor a double bond. Rather, all of the bonds are a hybrid of a single and double bond. In benzene, the pi bonding electrons are free to move completely around the ring. Delocalized electrons are electrons that are not confined to the bond between two atoms, but are instead allowed to move between three or more. The delocalization of the electrons in benzene can best be shown by showing benzene with a ring inside the hexagon, with the hydrogen atoms understood.
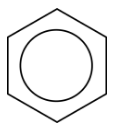
Delocalization of the electrons makes for a more stable molecule than a similar molecule that does not have delocalized electrons. Benzene is a more stable and less reactive compound than straight-chain hexenes. The \(sp^2\) hybridization of the carbon atoms results in a planar molecule as opposed to the puckered structure of cyclohexane.
There are many derivatives of benzene. The hydrogen atoms can be replaced by many different substituents. Aromatic compounds more readily undergo substitution reactions than addition reactions; replacement of one of the hydrogen atoms with another substituent will leave the delocalized double bonds intact. The following are typical examples of substituted benzene derivatives:
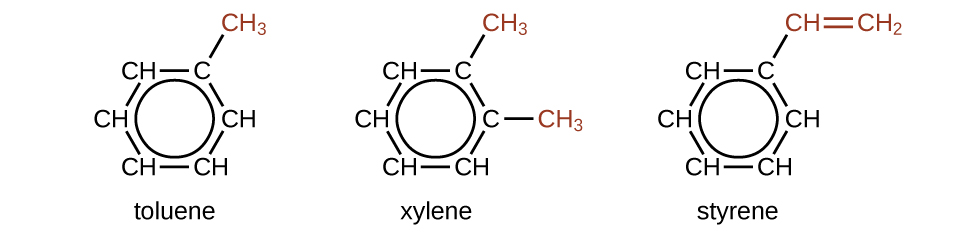
Toluene and xylene are important solvents and raw materials in the chemical industry. Styrene is used to produce the polymer polystyrene. The figure below shows the structural formulas for vanillin and naphthalene. Naphthalene is a chemical which is commonly used in mothballs. The vanillan molecule is extracted from vanilla beans, and gives vanilla it's distinctive aroma.

Figure \(\PageIndex{4}\) (left) Vanillin molecule ; (right) naphthalene. Vanilla_naphthalene © 2024 by Jason D'Acchioli is licensed under CC BY-NC-SA 4.0
Nomenclature of Aromatic Compounds
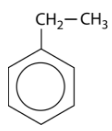
The simplest aromatic compounds are benzene rings with one substituent replacing one of the hydrogen atoms. If this substituent is an alkyl group, it is named first, followed in one word with "benzene". The molecule shown below is therefore called ethylbenzene.
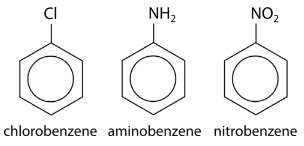
Substituents can be groups other than alkyl groups. If a chlorine atom were substituted for a hydrogen, the name becomes chlorobenzene. An \(\ce{-NH_2}\) group is called an amino group, so the corresponding molecule is called aminobenzene, often referred to as an aniline. An \(\ce{-NO_2}\) group is called a nitro group and so the third example below is nitrobenzene.
If more than one substituent is present, their location relative to each other can be indicated by numbering the positions on the benzene ring.
The number of the carbon location then precedes the name of the substituent in the overall name, with the numbers separated by a comma. As with branched alkanes, the system requires that the numbers be the lowest possible and that prefixes be used for more than one of the same substituent. If there are different substituents, the first in alphabetical order is given the lower number and listed first. The structures below are called 1,2-dimethylbenzene and 1-ethyl-4-methylbenzene.
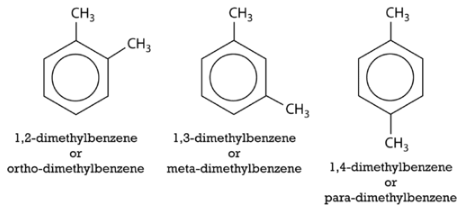
An alternate system for naming di-substituted benzene rings uses three different prefixes: ortho, meta, and para. If two groups are in the ortho position, they are on adjacent carbon atoms. The meta positioning refers to being in a 1,3 arrangement. The para positioning refers to being in a 1,4 arrangement. Shown below are the three possibilities for dimethylbenzene, also called xylene.
Lastly, a benzene ring missing one hydrogen atom \(\left( \ce{-C_6H_5} \right)\) can itself be considered the substituent on a longer chain of carbon atoms. That group is called a phenyl group and so the molecule below is called 2-phenylbutane.

Summary
- Aromatic hydrocarbons contain ring structures with delocalized π electron systems.
- Benzene is the parent compound of the large family of organic compounds known as aromatic compounds.
- Naming of aromatic compound i.e. benzene rings with different substituents is described.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).

