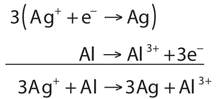8.2: Half-Reactions and Balancing Redox Equations
- Page ID
- 488928
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Balancing Redox Equations using Half-reactions
Another way to balance redox reactions is by the half-reaction method. This technique involves breaking an equation into its two separate components - the oxidation half reaction and the reduction half reaction. Since neither oxidation nor reduction can actually occur without the other, we refer to the separate equations as half-reactions.
The general technique involves the following:
- The overall equation is broken down into two half-reactions. If there are any spectator ions, they are removed from the equations.
- Each half-reaction is balanced separately - first for atoms and then for charge. Electrons are added to one side of the equation or the other in order to balance charge. For example, if the reactant side of the equation has a total charge of +3, the product side must also equal +3.
- Next the two equations are compared to make sure electrons lost equal electrons gained. One of the half reactions will be an oxidation reaction, the other will be a reduction reaction.
- Finally the two half-reactions are added together, and any spectator ions that were removed are placed back into the equation
Consider the following reaction:
\[ \ce{Mg(s) + Cl2(g) -> MgCl2(s)} \nonumber \]
In this reaction, Mg is oxidized and Cl is reduced. You may find it useful to use oxidation numbers to help you determine this. Mg changes from 0 to +2; Cl changes from 0 to -1.
When we write the half-reactions,we break apart compounds that contain either of the key elements (elements undergoing oxidation or reduction). Oxidation numbers are written as if they were ion charges. Notice that the chlorine from MgCl2 is written as two separate ions, not combined as is Cl2. Balance the two reactions for atoms.
| \[\ce{Mg → Mg^{2+}} \nonumber \] | \[\ce{Cl2 → 2 Cl-} \nonumber \] |
Next balance the equations for charge by adding electrons. Remember - one half-reaction will be an oxidation reaction (electrons on the product side) and the other will be reduction (electrons will be on the reactant side)
| \[ \ce{Mg -> Mg^{2+} + 2 e-} \nonumber \] | \[\ce{Cl2 + 2 e- -> 2 Cl^{-}} \nonumber \] |
| oxidation | reduction |
In this example, balancing for charge results in both sides, of both equations, having net charges of 0. That won't always be the case. Be sure you see in this example how charges are balanced.
We then compare the two equations for numbers of electrons. We see that both equations have 2 electrons so we do not need to make any adjustments for that. Finally, add the two equations together:
\[\ce{Mg + Cl2 -> Mg^{2+} + 2 Cl^{-}} \nonumber \]
and reform any compounds broken apart in the earlier steps:
\[\ce{Mg + Cl2 -> MgCl2} \nonumber \]
We see that the original equation was already balanced, not just for atoms but for electrons as well.
\[\ce{Cu(s) + AgNO3(aq) -> Cu(NO3)2 (aq) + Ag(s)} \]
Solution
Identify the elements undergoing oxidation (Cu) and reduction (Ag). The nitrate polyatomic anion (NO3-) is a spectator ion which we will not include in our half-reactions.
| \[\ce{Cu -> Cu^{2+} + 2 e-} \nonumber \] | \[ \ce{Ag+ + 1 e- -> Ag} \nonumber \] |
| oxidation | reduction |
After balancing for atoms and for charge, we see that the two equations do not have the same number of electrons - there are 2 in the copper reaction but only one in the silver reaction. Multiply everything in the silver reaction by 2, then we will add the equations together:
| Step 1 | Step 2 | Step 3 |
|
|
||
| Write the balanced half-reactions |
Balance electrons | Add half-reactions |
| \[\ce{Cu -> Cu^{2+} + 2 e-} \nonumber\] | \[\ce{Cu -> Cu^{2+} + \bcancel{2 e-}} \nonumber\] | |
| \[\ce{Ag+ + 1e- -> Ag} \nonumber\] | × 2 | \[\ce{2Ag+ + \bcancel{2e-} -> 2Ag} \nonumber\] |
|
|
||
| Add equations together | \[\ce{Cu + 2Ag+ -> Cu^{2+} + 2 Ag} \nonumber \] | |
| Reform compound/return spectator ions if necessary | \[\ce{Cu + 2 AgNO3 → Cu(NO3)2 + 2 Ag} \] | |
Write and balance the redox reaction that has silver ions and aluminum metal as reactants and silver metal and aluminum ions as products, expressed by the following chemical reaction.
\[\ce{Ag^{+}(aq) + Al(s) -> Ag(s) + Al^{3+}(aq)}\]
- Answer
-
\[\ce{Ag^{+} + Al → Ag + Al^{3+}} \nonumber \]
The equation looks balanced as it is written. However, when we compare the overall charges on each side of the equation, we find a charge of +1 on the left but a charge of +3 on the right. This equation is not properly balanced. To balance it, let us write the two half reactions. Silver ions are reduced, and it takes one electron to change Ag+ to Ag:
\[\ce{Ag^{+} + e^{−} → Ag} \nonumber \]
Aluminum is oxidized, losing three electrons to change from Al to Al3+:
\[\ce{Al → Al^{3+} + 3e^{−}} \nonumber \]
To combine these two half reactions and cancel out all the electrons, we need to multiply the silver reduction reaction by 3: