3.30: Quiz 6A Key
- Page ID
- 19334
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Clearly indicate true (T) or false (F) for the following statements (2 pts each):
| __F__ | Enantiomers of a compound will have different boiling points. |
| __T__ | A chiral catalyst or enzyme can be used to synthesize a single enantiomer product. |
| __T__ | A primary carbon is less sterically hindered than a tertiary carbon, which explains why a primary alkyl halide reacts faster than a tertiary alkyl halide in an SN2 reaction. |
- (2 pts each) Multiple Choice/fill-in-the-box (choose the best answer from the options given):
- Which of the following reactions will give a racemic product?
 An achiral Rh metal complex for the hydrogenation reaction of an alkene
An achiral Rh metal complex for the hydrogenation reaction of an alkene- A reaction with the enzymes in Bakerʼs yeast
- An enzyme that only recognizes a trans alkene substrate
- A chiral Rh metal complex for the hydrogenation reaction of an alkene
- What is the chemical reason for the controversy regarding the use of methyl iodide as an agricultural fumigant?
 Methyl iodide is a racemic molecule and one enantiomer has toxic side-effects.
Methyl iodide is a racemic molecule and one enantiomer has toxic side-effects.- Methyl iodide catalyzes the hydrolysis reaction of DNA, making it toxic.
- Methyl iodide is an electrophile that will alkylate DNA, making it toxic.
- Methyl iodide makes strawberries taste bad.
- Stereoisomers with an asymmetric carbon center and non-superimposable mirror images are:
 cis/trans isomers
cis/trans isomers- enantiomers
- constitutional isomers
- end-of-the-quarter isomers
- regioisomers
- A 50:50 mixture of two enantiomers is called a ____________
 memorial day mixture
memorial day mixture- cis/trans mixture
- mirror image
- constitutional mixture
- racemic mixture
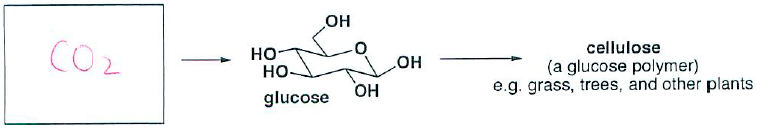
- (2 pts) What carbon building block does nature use to synthesize the following product(s)?
- Which of the following reactions will give a racemic product?
-
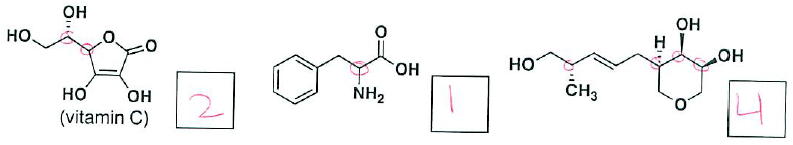
- (6 pts) How many asymmetric carbon centers does each of the following molecules have?
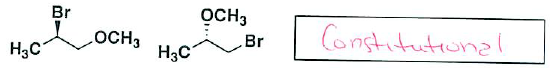
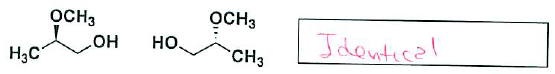
- (6 pts) Indicate for the following pairs of compounds whether they are identical, constitutional isomers, or enantiomers. (put your answer in the box provided – you may use an answer more than once, or not at all)
-
-
- (2 pts) Provide a brief definition indicating what factors make a molecule a good nucleophile.
The molecule should have high electron density (π bonds, lone pairs) that is readily available to make new bonds (delocalized e- is less available).
- (4 pts) Circle which molecule is a better nucleophile and briefly explain your answer.
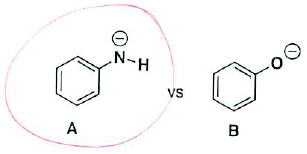
Nitrogen is less electronegative than oxygen, therefore it won't stabilize the negative charge as well, making A a better nucleophile.
-
- (5 pts) Identify the asymmetric carbon center in the molecule shown below and then draw a perspective structure for each enantiomer.

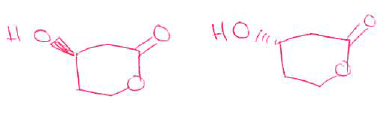
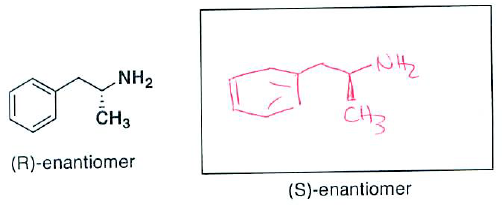
- (3 pts) The (R)-enantiomer of an amine molecule is shown below. Draw the perspective structure for the (S)-enantiomer (ie, the other enantiomer) in the box provided.
- (5 pts) Identify the asymmetric carbon center in the molecule shown below and then draw a perspective structure for each enantiomer.
- Extra Practice:
- (4 pts) Draw the racemic mixture (both enantiomers) that result upon hydrogenation of the following alkene with an achiral catalyst.
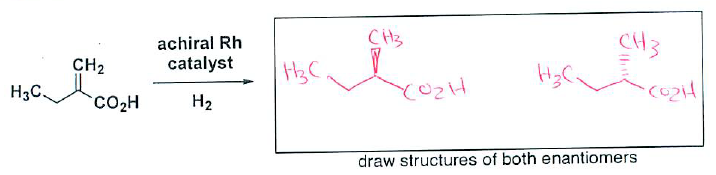
- (2 pts) How would you expect the reaction to be different if a chiral Rh catalyst is used?
You would form only one enantiomer.