4.7: Types of Chemical Reactions
- Page ID
- 34028
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Explain the following laws within the Ideal Gas Law:
- Avogadro's law of gases
The chemical reactions we have described are only a tiny sampling of the infinite number of chemical reactions possible. How do chemists cope with this overwhelming diversity? How do they predict which compounds will react with one another and what products will be formed? The key to success is to find useful ways to categorize reactions. Familiarity with a few basic types of reactions will help you to predict the products that form when certain kinds of compounds or elements come in contact. Most chemical reactions can be classified into one or more of five basic types. The general forms of these five kinds of reactions are summarized in Table 3.2.1, along with examples of each.
Table 4.7.1: Basic Types of Chemical Reactions
| Name of Reaction | General Form | Example(s) |
|---|---|---|
|
oxidation–reduction (REDOX) reactions. |
oxidant + reductant → reduced oxidant + oxidized reductant | C7H16(l) + 11O2(g) → 7CO2(g) + 8H2O(g) |
| acid–base reactions | acid + base → salt | NH3(aq) + HNO3(aq) → NH4+(aq) + NO3−(aq) |
|
exchange reactions |
AB + C → AC + B | CH3Cl + OH− → CH3OH + Cl− |
| AB + CD → AD + CB | BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq) | |
|
condensation reactions |
A + B → AB | CO2(g) + H2O(l) → H2CO3(aq) |
| HBr + H2C=CH2 → CH3CH2Br* | ||
| cleavage reactions | AB → A + B | CaCO3(s) → CaO(s) + CO2(g) |
| CH3CH2Cl → H2C=CH2 + HCl** | ||
| * In more advanced chemisty courses you will learn that this reaction is also called an addition reaction. | ||
| ** In more advanced chemistry courses you will learn that this reaction is also called an elimination reaction. | ||
It is important to note, however, that many reactions can be assigned to more than one classification, as you will see in our discussion. The classification scheme is only for convenience; the same reaction can be classified in different ways, depending on which of its characteristics is most important. Oxidation–reduction reactions, in which there is a net transfer of electrons from one atom to another, and condensation reactions are discussed in this section. Acid–base reactions and one kind of exchange reaction—the formation of an insoluble salt such as barium sulfate when solutions of two soluble salts are mixed together.
Note
Many reactions can be assigned to more than one classification.
Oxidation-reduction (redox) reaction
A redox reaction occurs when the oxidation number of atoms involved in the reaction are changed. Oxidation is the process by which an atom’s oxidation number is increased, and reduction is the process by which an atom’s oxidation number is decreased. If the oxidation states of any elements in a reaction change, the reaction is an oxidation-reduction reaction. An atom that undergoes oxidation is called the reducing agent, and the atom that undergoes reduction is called the oxidizing agent. An example of a redox reaction is the reaction between hydrogen gas and fluorine gas:
\[H_2 (g) + F_2 (g) \rightarrow 2HF (g)\]
In this reaction, hydrogen is oxidized from an oxidation state of 0 to +1, and is thus the reducing agent. Fluorine is reduced from 0 to -1, and is thus the oxidizing agent.
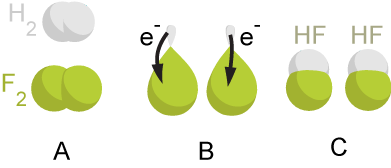
Figure 2.2: In this redox reaction, a \(H_2\) molecule donates electrons to \(F_2\) resulting in two \(HF\) molecules
A combustion reaction is a type of redox reaction during which a fuel reacts with an oxidizing agent, resulting in the release of energy as heat. Such reactions are exothermic, meaning that energy is given off during the reaction. An endothermic reaction is one which absorbs heat. A typical combustion reaction has a hydrocarbon as the fuel source, and oxygen gas as the oxidizing agent. The products in such a reaction would be \(CO_2\) and \(H_2O\).
\[C_xH_yO_z+O_2 \rightarrow CO_2+H_2O \;\;\; \text{(unbalanced)}\]
Such a reaction would be the combustion of glucose in the following (unbalanced) equation:
\[C_6H_{12}O_6 (s) + O_2 (g) \rightarrow CO_2 (g) +H_2O (g)\]
Real life example: explosion; burning
Acid-base (Neutralization) Reaction
A neutralization reaction occurs when an acid and base are mixed together. An acid is a substance that produces H+ ions in solution, whereas a base is a substance that that produces OH- ions in solution. A typical acid-base reaction will produce an ionic compound called a salt and water. A typical acid-base reaction is the reaction between hydrochloric acid and sodium hydroxide. This reaction is represented by the equation:
\[HCl (aq) + NaOH (aq) \rightarrow NaCl (aq)+ H_2O (l)\]
In this reaction, \(HCl\) is the acid, \(NaOH\) is the base, and \(NaCl\) is the salt.
Real life example: Baking soda reacts with vinegar is a neutralization reaction.
Condensation Reaction
A condensation reaction occurs when one or more compounds combines to form a complex compound. The simplest equation of synthesis reaction is illustrated below.
.png?revision=2)
An example of such a reaction is the reaction of silver with oxygen gas to form silver oxide:
\[2Ag (s) +O_2 (g) \rightarrow 2AgO (s)\]
Real life example: Hydrogen gas is burned in air (reacts with oxygen) to form water:
\[2H_2(g) + O_2(g) \rightarrow 2H_2O(l)\]
Cleavage Reaction
A cleavage reaction is the opposite of a synthesis reaction. During a cleavage reaction, a more complex compound breaks down into multiple simpler compounds. A classic example of this type of reaction is the decomposition of hydrogen peroxide \(H_2O_2\) into oxygen \(O_2\) and hydrogen gas \(H_2\):
\[H_2O_2 (l) \rightarrow H_2 (g) + O_2 (g)\]

Figure 2.4: The molecule AB is decomposing into A and B
Real life examples: Electrolysis of water; Carbonic acid in soda
Exchange Reaction (Single Replacement)
A type of oxidation-reduction reaction in which an element in a compound is replaced by another element.
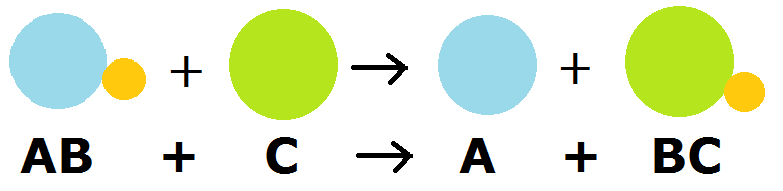.png?revision=2)
An example of such a reaction is:
\[Cu (s) + AgNO_3 (aq) \rightarrow Ag(s) + Cu(NO_3)_2 (aq)\]
Exchange Reaction (Double Replacement)
A reaction that occurs when aqueous solutions of anions (negatively charged ions) and cations (positively charged ions) combine to form a compound that is insoluble is known as precipitation. The insoluble solid is called the precipitate, and the remaining liquid is called the supernate. See Figure 4.7.1
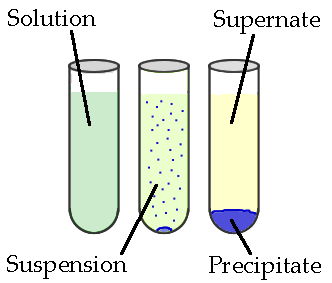
Figure 4.7.1
Real life example: The white precipitate formed by acid rain on a marble statue:
\[CaCO_3(aq)+H_2SO_4(aq) \rightarrow CaSO_4(s)+H_2O(l)+CO_2(g)\]
Example 2.1: Precipitation
An example of a precipitation reaction is the reaction between silver nitrate and sodium iodide. This reaction is represented by the chemical equation :
AgNO3 (aq)+ NaI (aq) → AgI (s) + NaNO3 (aq)
Since all of the above species are in aqueous solutions, they are written as ions, in the form:
Ag+ +NO3- (aq)+ Na+ (aq) + I- (aq) → AgI (s) + Na+ (aq) + NO3- (aq)
Ions that appear on both sides of the equation are called spectator ions. These ions do not affect the reaction and are removed from both sides of the equation to reveal the net ionic equation, as written below:
Ag+ (aq) + I- (aq) → AgI (s)
In this reaction, the solid, AgI, is known as the precipitate. The formation of a precipitate is one of the many indicators that a chemical reaction has taken place.
Problems
a) Al(OH)3 (aq) + HCl (aq) → AlCl3 (aq) + H2O (l)
b) MnO2 + 4H+ + 2Cl- → Mn2+ + 2H2O (l) + Cl2 (g)
c) P4 (s) + Cl2 (g) → PCl3 (l)
d) Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
e) AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
Solutions
5a) Acid-base
5b) Oxidation-reduction
5c) Synthesis
5d) Single-replacement reaction
5e) Double replacement reaction

