5.3 Radical Bromination of Alkenes Part II: Allylic Bromination with NBS
- Page ID
- 449192
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write the equation for the bromination of a symmetrical alkene using N-bromosuccinimide.
- predict the product formed when a given symmetrical alkene is treated with N-bromosuccinimide.
- identify the reagent, the symmetrical alkene, or both, needed to produce a given allyl halide by allylic bromination.
- list the following radicals in order of increasing or decreasing stability: allyl, vinyl, primary alkyl, secondary alkyl, tertiary alkyl, methyl.
- explain the ease of forming an allyl radical, and the difficulty of forming a vinyl radical, in terms of relative C–H bond dissociation energies.
We have discussed the electrophilic addition of X2 and HX to alkenes as a route to forming alkyl halides in CHM 222. In this section we introduce bromination at the allyic position with N-bromosuccinimide (NBS). Notice that at the moment we are restricting our studies to the allylic bromination of symmetrical alkenes, such as cyclohexene. When we introduce an element of asymmetry, we find that more than one allyl radical can be formed; therefore, we must assess the relative stability of each radical when trying to predict which product will predominate. The method of doing this assessment is described in the next section.
Allylic Bromination
Previously, alkyl halides have been produced though reactions with alkenes. Hydrogen halides (HCl, HBr, and HI) react with alkenes in an electrophilic addition reaction to yield alkyl halides as products. Also, Bromine (Br2) and chlorine (Cl2) can react with alkenes to provide dihalogenated products.
Another method for preparing alkyl halides from alkenes is with N-bromosuccinimide (NBS) in carbon tetrachloride (CCl4) solution with the presence of light. The reaction specifically causes the substitution of bromine with a hydrogen attached to a carbon adjacent to the double bond - the allylic position.
General Reaction
Mechanism
The allylic bromination with NBS occurs as a radical chain reaction. NBS is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts very rapidly with the HBr formed during the reaction mechanism to provide bromine (Br2) which is required for the reaction to continue. Under the correct conditions, NBS provides a constant but very low concentration of Br2 in the reaction mixture. The low concentration of Br2 helps to prevent the formation of unwanted side-products.
The mechanism starts with the formation of a small amount of bromine radical which then abstracts an allylic hydrogen to form an allylic radical and HBr. The HBr can then react with NBS to form the Br2 required for the reaction. The allylic radical then abstracts a bromine atom from Br2 to form the allyl halide product and a bromine radical. The bromine radical produced allows the reaction to continue.
The predominance of allylic substitution over other positions is based on bond dissociation energies. An allylic C-H bond has a strength of about 88 kcal/mol which is much weaker than a typical alkyl C-H bond (98 kcal/mol) or vinylic C-H bond (111 kcal/mol). Therefore, an allylic C-H bond is most likely to form a free radical and react.
Because a allylic C-H bond requires less energy to undergo homolytic cleavage than even a tertiary C-H bond, it can be inferred that a allylic radical is more stable than a tertiary radical. The ordering of stability in radicals can be expanded to include vinylic and allylic radicals. The enhanced stability of allyl radicals can be attributed to resonance stabilization which will be discussed in the next section.

Benzylic and allylic radicals are more stable than alkyl radicals due to resonance effects - an unpaired electron can be delocalized over a system of conjugated pi bonds. An allylic radical, for example, can be pictured as a system of three parallel 2pz orbitals sharing three electrons. With two resonance forms, the allylic radical is electronically symmetrical. Due to resonance hybrid theory, neither structure is correct, but instead the structure lies somewhere between the two resonance forms. Another way to phrase this is that the unpaired electron is delocalized across all the carbon atoms through the pi system and not localized on one site. The more resonance structures, the more stable the molecule. This is why the allylic radical is more stable than the alkyl radical.
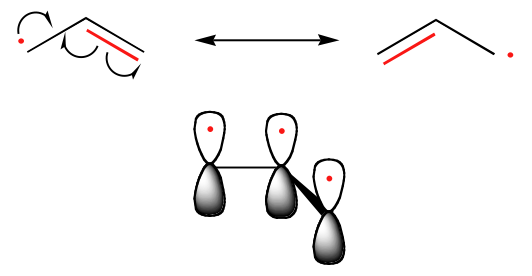
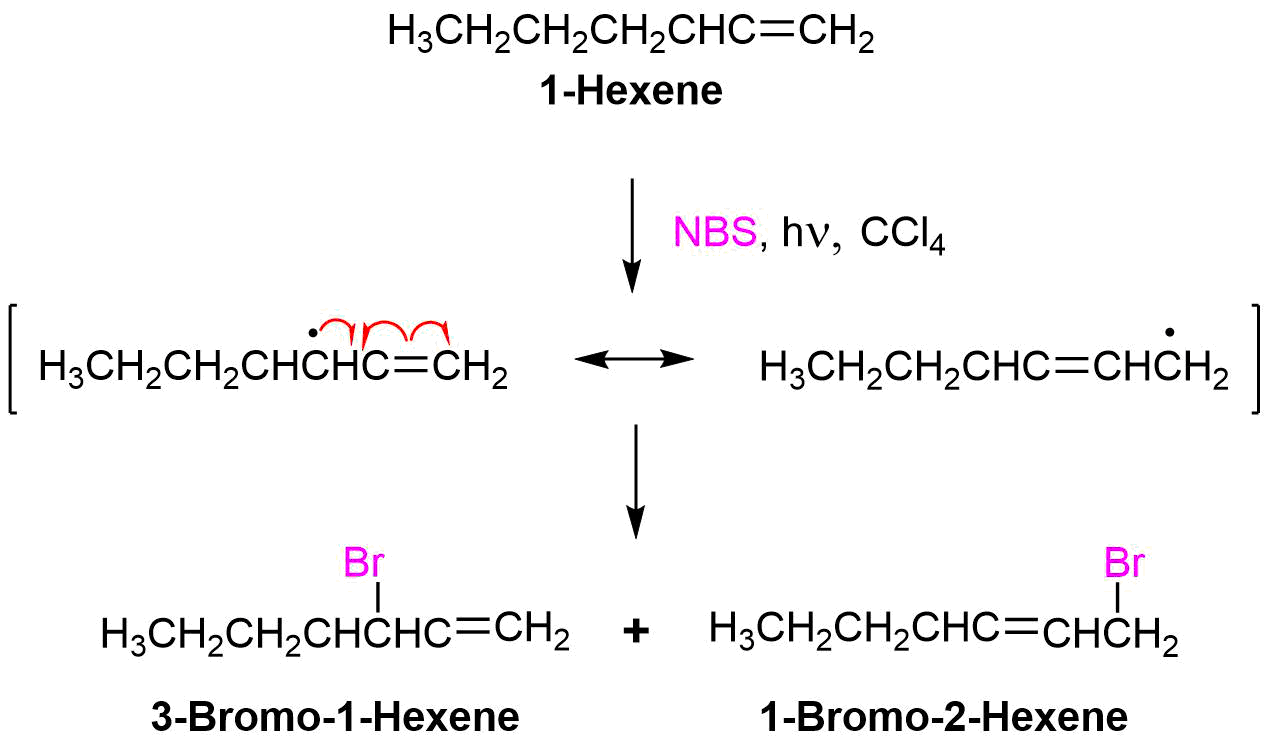
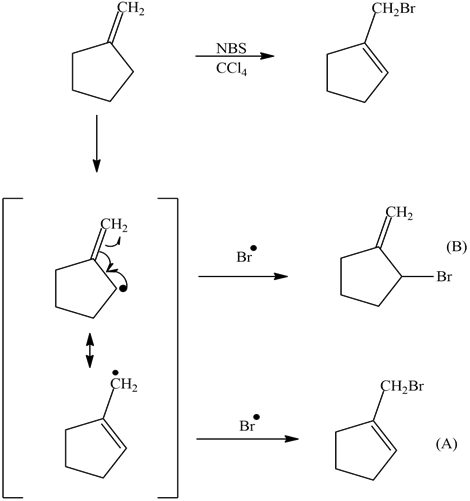
Because the allylic radical is symmetrical, a reaction can occur on either side. Therefore if reacting with bromine, the bromination could occur on either end of the allylic radical. When the allyl radical is symmetrical, this yields the same product. However, if you have an unsymmetrical allyl radical, it would lead to a mixture of products and not necessarily in equal amounts. This is because the intermediate radical is unsymmetrical. The reaction will occur at the less hindered site. An example would be 1-octene as a starting material in a bromination. The products the reaction would yield would be 3-bromo-1-octene and 1-bromo-2-octene.
Further Reactions
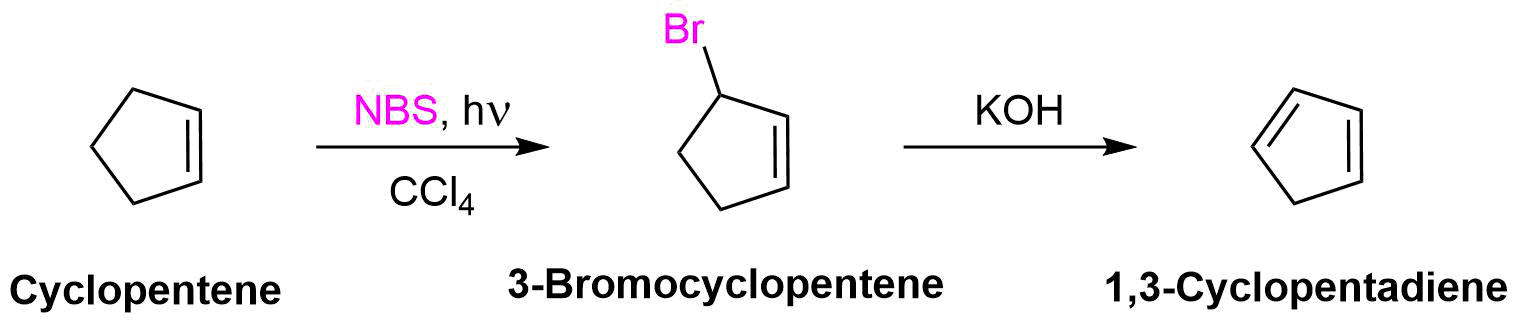
The products from allylic bromination reactions can easily be converted into dienes by elimination using a base.
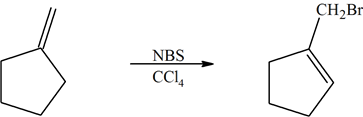
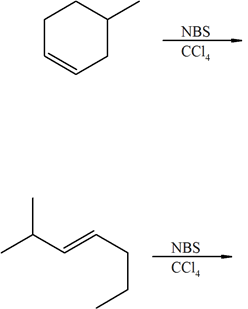
1) The following reaction shows the major product. Explain why this would be the final product and why the 2° bromo product is not the major product.
2) Predict the products of the following reactions:
- Answer
-
1) The product (A) is a 1° halogen which is more predominant product even though the (B) had a better transition state with a 2° radical. The 1° radical intermediate is not as sterically hindered.
2)

