21.5: Hydrolysis of nitriles
- Page ID
- 225900
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
A nitrile contains a triply bonded C=N group, which is another common type of polar bond. However, the carbon in a nitrile is at an oxidation state of +3, higher than the +2 for ketones or +1 for aldehydes. For this reason, nitriles are not carbonyl equivalents like imines; rather, they are at the same oxidation state as carboxylic acids.
The C=N triple bond undergoes nucleophilic additions in a similar way to a C=O. As with the carbonyl group, so often protonation is needed in order to activate it for the addition of weak nucleophiles such as water. As expected, when nitriles are hydrolyzed (by nucleophilic addition of water followed by nucleophile elimination), they form either carboxylic acids or amides, depending on the conditions.
General reaction

Water is used along with the acid, to provide the reagent for the hydrolysis.
Example
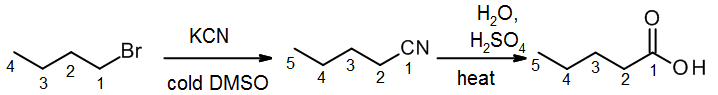
Since nitriles can be prepared from alkyl halides + cyanide ion via an SN2 reaction, this hydrolysis step can be used to provide a two step synthesis sequence for making a carboxylic acid with one additional carbon. In this example, a four carbon alkyl halide is converted to a five carbon carboxylic acid.
Contributors
Prof. Steven Farmer (Sonoma State University)
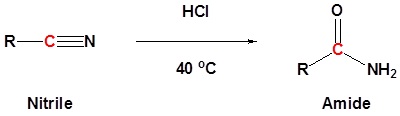
Conversion of nitriles to amides
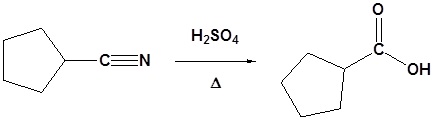
General reaction
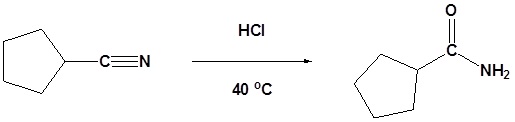
Example
Mechanism
1) Protonation

2) Nucleophilic attack by water
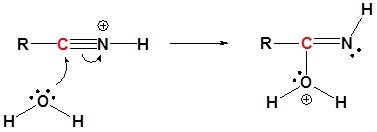
3) Proton Transfer

4) Resonance
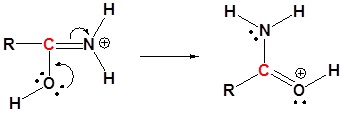
5) Deprotonation
Contributors
- Prof. Steven Farmer (Sonoma State University)
- Hydrolysis of nitriles. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. License: CC BY-SA: Attribution-ShareAlike
- Conversion of nitriles to amides. Authored by: Prof. Steven Farmer . Provided by: Chemistry LibreTexts. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Nitriles/Reactivity_of_Nitriles/Conversion_of_nitriles_to_amides. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 17.19 The Hydrolysis of Nitriles. Authored by: Prof. Steve Farmer. Provided by: Chemistry LibreTexts. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Bruice)/17%3A_Carbonyl_Compounds_I%3A_Reactions_of_Carboxylic_Acids_and_Carboxylic_Derivatives/17.19____The_Hydrolysis_of_Nitriles. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike