9.5: Endothermic and Exothermic Reactions
- Page ID
- 313282
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Endothermic and Exothermic Reactions
Endothermic and exothermic reactions can be thought of as having energy as either a reactant or a product. Endothermic reactions require energy, so energy is a reactant. Heat flows from the surroundings to the system (reaction mixture) and the energy of the system increases. In an exothermic reaction, heat is released (considered a product) and the energy of the system decreases.
In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets cold). A chemical reaction is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from the system, the temperature of the surroundings increases.
Endothermic Reaction: When \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: \text{kJ}\) of heat is absorbed. Because the heat is absorbed by the system, the \(177.8 \: \text{kJ}\) is written as a reactant.

Exothermic Reaction: When methane gas is combusted, heat is released, making the reaction exothermic. Specifically, the combustion of \(1 \: \text{mol}\) of methane releases 890.4 kilojoules of heat energy. The amount of heat released is therefore written on the product side of the reaction.
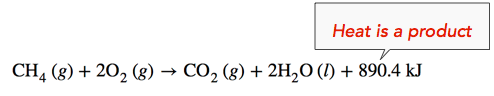
Example \(\PageIndex{1}\)
Is each chemical reaction exothermic or endothermic?
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 213 kcal
- N2(g) + O2(g) + 45 kcal → 2NO(g)
Solution
- Because energy (213 kcal) is a product, energy is given off by the reaction. Therefore, this reaction is exothermic.
- Because energy (45 kcal) is a reactant, energy is absorbed by the reaction. Therefore, this reaction is endothermic.
Exercise \(\PageIndex{2}\)
Is each chemical reaction exothermic or endothermic?
- H2(g) + F2(g) → 2HF (g) + 130 kcal
- 2C(s) + H2(g) + 5.3 kcal → C2H2(g)
- Answer
-
a. The energy (130 kcal) is produced, hence the reaction is exothermic
b. The energy (5.3 kcal) is supplied or absorbed to react, hence, the reaction is endothermic
Energy Diagrams
Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the energy of the reactants is lower than that of the products. This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\). For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.
In an exothermic reaction, the energy of the products is lower than the energy of the reactants, hence is energetically downhill, shown in Figure \(\PageIndex{2B}\). Energy is given off as reactants are converted to products. The energy given off is usually in the form of heat (although a few reactions give off energy as light). In the course of an exothermic reaction, heat flows from the system to its surroundings, and thus, gets warm.
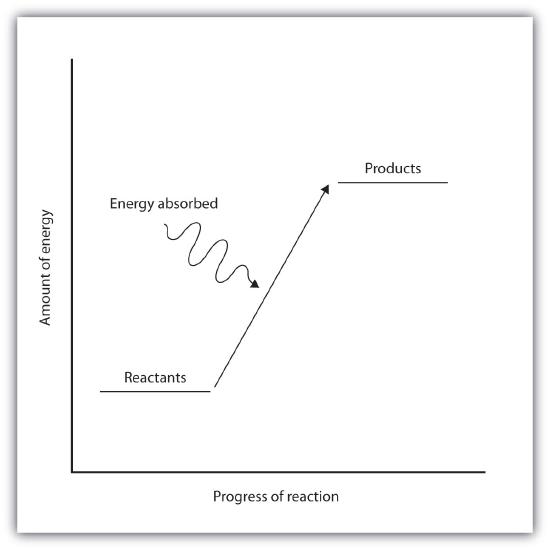
Figure \(\PageIndex{2A}\): Endothermic Reactions
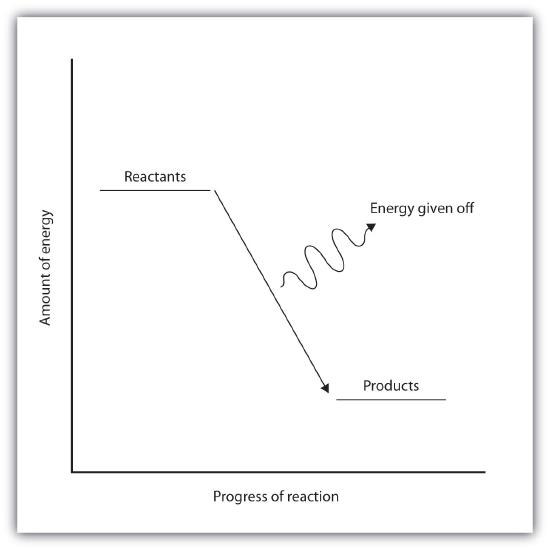
Table \(\PageIndex{2}\): Endothermic and Exothermic Reactions
| Endothermic Reactions | Exothermic Reactions |
| Heat is absorbed by reactants to form products. | Heat is released. |
| Heat is absorbed from the surroundings; as a result, the surroundings get cold. | Heat is released by the reaction to surroundings; surroundings feel hot. |
| The reactants are lower in energy than the products | The products are lower in energy than the reactants |
| Represented by an "uphill" energy diagram | Represented by an "downhill" energy diagram |
Thermochemical Stoicheometry
It is also possible to combine our previous studies of stoicheometry with thermochemical equations in order to relate the amount of energy released or absorbed during a reaction to the mass of product reacted or he mass of reactant created. The following two examples illustrate this.
Example \(\PageIndex{2}\)
How much energy is needed to react 72.3 g of carbon in the presence of excess hydrogen? Acetylene, C2H2, is produced as the product.
2C(s) + H2(g) + 5.3 kcal → C2H2(g)
Solution
If we treat the 5.3 kcal that appears in the chemical equation as another reactant, we can use it to form a stoichiometric ratios. This ratio can then be used in a dimentional analysis problem to find the solution. Don't forget to include the stoicheometric coefficient of 2 that appears in front of the carbon.
\(\mathrm{72.3\: g\: C\times\dfrac{1\: mol\: C}{12.01\: g\: C}\times\dfrac{5.3\: kcal}{2\: mol\: C} =16.0\: kcal}\)31.9 kcal of energy is therefore needed to react the 72.3 g of carbon.
Example \(\PageIndex{3}\)
Nitrogen monoxide is a pollutant produced as a byproduct whenever oxygen and nitrogen are heated together at a very high temperature. What mass of nitrogen monoxide is created when 27.51 kcal of energy is added to a mixture of oxygen and nitrogen?
N2(g) + O2(g) + 45 kcal → 2NO(g)
Solution
\(\mathrm{27.51\: kcal\times\dfrac{2\: mol\: NO}{45\: kcal}\times\dfrac{30.01\: g\: NO}{1\: mol\: NO}=36.69\: g\: NO}\)The formation of carbon monoxide is generally an undesired side reaction. One purpose of the catalytic converter in cars and trucks is to get rid of some of the NO created in the engine as a byproduct of hydrocarbon combustion.
Exercises
-
Is each chemical reaction exothermic or endothermic?
- 2SnCl2(s) + 33 kcal → Sn(s) + SnCl4(s)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 213 kcal
-
Is each chemical reaction exothermic or endothermic?
- C2H4(g) + H2(g) → C2H6(g) + 137 kJ
- C(s, graphite) + 1.9 kJ → C(s, diamond)
Answers
1. a: endothermic, b: exothermic
2. a: exothermic, b: endothermic

