3.9 Exceptions to the Octet Rule
- Page ID
- 142208
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
-
To assign a Lewis dot symbol to elements not having an octet of electrons in their compounds.
Three cases can be constructed that do not follow the octet rule, and as such, they are known as the exceptions to the octet rule. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. However, it is hard to imagine that one rule could be followed by all molecules. There is always an exception, and in this case, three exceptions:
- When there are an odd number of valence electrons
- When there are too few valence electrons
- When there are too many valence electrons
Exception 1: Species with Odd Numbers of Electrons
The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be nitrogen monoxide also called nitric oxide (\(\ce{NO}\).
- (1 O atom) x (8) + (1 N atom) x (8) = 16 valence electrons needed
- (1 O atom) x (6) + (1 N atom) x (5) = 11 valence electrons present.
- 16 - 11 = 5 electrons short, thus 5 electrons must be shared
- 5 shared electrons/ 2 electrons per bond = 2.5 bonds How can you have a half of a bond? You can't according to Lewis dot structure rules. Instead, draw a structure with two bonds (essentially ignoring the 1/2 bond).
- The two bonds have to be placed between the N and O atoms.
N=O
6. You have used up 4 of the 11 electrons. Add in the remaining seven as 3 lone pairs and one unpaired electron. But where should the lone pairs and the one unpaired electron go? The unpaired electron is usually placed in the Lewis Dot Structure so that each element in the structure will have the lowest formal charge possible. Oxygen normally has six valence electrons. If we give the O atom two lone pairs of electrons, and it participates in two bonds (a double bond) with nitrogen, the O atom will have a formal charge of 0. Nitrogen normally has five valence electrons. If the N atom participates in two bonds with oxygen and is assigned one lone pair along with one single electron, the formal charge on the N atom will be 0. The O atom will have an octet of valence electrons, but the N atom will have only seven valence electrons. That is acceptable because this molecule is an odd-elctron particle and one atom in the molecule must have fewer than eight valence electrons.
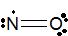
Figure \(\PageIndex{}\): The proper Lewis structure for NO molecule
Free Radicals
There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired electrons. Most odd electron species, which we call free radicals, are highly reactive. Because of their instability, free radicals bond to atoms in which they can take an electron from in order to become stable, making them very chemically reactive. Radicals are found as both reactants and products, but generally react to form more stable molecules as soon as they can. In order to emphasize the existence of the unpaired electron, radicals are denoted with a dot in front of their chemical symbol as with \(\cdot OH\), the hydroxyl radical. An example of a radical you may by familiar with already is the gaseous chlorine atom, denoted \(\cdot Cl\).
Exception 2: Incomplete Octets - Carbocations
The second exception to the octet rule is when there are too few valence electrons on one atom, which results in an incomplete octet. Species with incomplete octets are pretty rare and generally are only found in some beryllium, aluminum, and boron compounds including the boron hydrides. But they are also found in organic chemistry as reaction intermediates - species that are formed in one step of a reaction, but then used up in some following step. Let's take a look at one such carbocation, CH3CH2CH2+:
- (3 C atoms) x (8) + (7 H atoms) x (2) = 38 valence electrons needed
- (3 C atoms) x (4) + (7 H atoms) x (1) -1 = 18 valence electrons present.
- 38 - 18 = 20 electrons short, thus 20 electrons must be shared
- 20 shared electrons/ 2 electrons per bond = 10 bonds But, how can you have 10 bonds if you have only 18 electrons present? You can't . Instead, draw a structure with 9 bonds (subtract one bond from the number of bonds that you calculated.)
- You can draw single bonds between the C and H atoms, using up all 18 electrons, as shown here:
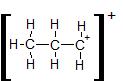
The problem with this structure is that C atom on the right has an incomplete octet; it only has six electrons around it. Hydrogen atoms can naturally only have only 2 electrons in their outermost shell (their version of an octet), and as such there are no spare electrons to form a double bond with carbon. A carbocation such as this is commonly a transitory species, formed temporarily in reactions that involve multiple steps.
Example \(\PageIndex{1}\): \(NF_3\)
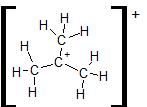
Draw the Lewis structure for (CH3)3C+
SOLUTION
- (4 C atoms) x (8) + (9 H atoms) x (2) = 50 valence electrons needed
- (4 C atoms) x (4) + (9 H atoms) x (1) -1 = 24 valence electrons present.
- 50 - 24 = 26 electrons short, thus 26 electrons must be shared
- 26 shared electrons/ 2 electrons per bond = 13 bonds But, how can you have 13 bonds if you have only 24 electrons present? You can't . Instead, draw a structure with 12 bonds (subtract one bond from the number of bonds that you calculated.)
- You can draw single bonds between the C and H atoms, using up all 24 electrons, as shown here:
Exception 3: Expanded Valence Shells
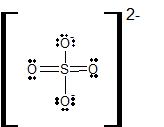
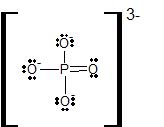
Compounds containing expanded valence shell atoms occur when the central atom in a Lewis structure has more than eight electrons in its valence shell. In expanded octets, the central atom can have ten, twelve, or even fourteen valence electrons. Particles with expanded octets involve a nonmetal central atom from the third row or below, and a highly electronegative terminal atom. We will not work with any of these particles, but it is important to note that two common polyatomic ions, SO42- and PO43-, can be drawn, in their most stable resonance form, with the S and P atoms as expanded valence shell atoms:
Summary
Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. There are three exceptions: (1) When there are an odd number of valence electrons, (2) When there are too few valence electrons, and (3) when there are too many valence electrons
References
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. G.; Madura, Jeffrey D. General Chemistry: Principles & Modern Applications. 9th Ed. New Jersey. Pearson Education, Inc. 2007.
- Moore, John W.; Stanitski, Conrad L.; Jurs, Peter C. Chemistry; The Molecular Science. 2nd Ed. 2004.
Contributors
- Modified by Tom Neils (Grand Rapids Community College)

