5.4: Ionizing Radiation and Non-ionizing Radiation
- Page ID
- 85161
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Qualitatively compare the ionizing and penetration power of alpha particles (α), beta particles (β), and gamma rays (γ).
- Describe the biological impact of ionizing radiation.
- Know the most common source of background radiation and how to minimize exposure to this source.
The Ionizing and Penetration Power of Radiation
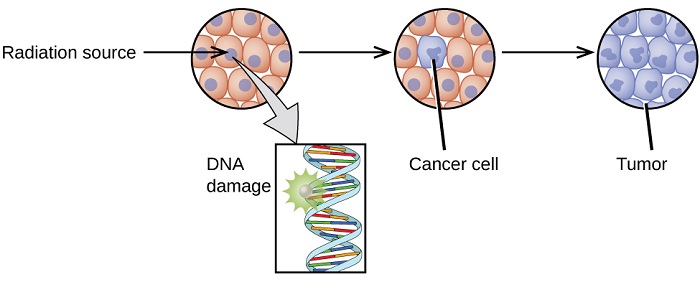
The increased use of radioisotopes has led to increased concerns over the effects of these materials on biological systems (such as humans). All radioactive nuclides emit high-energy particles or electromagnetic waves. When this radiation encounters living cells, it can cause heating, break chemical bonds, or ionize molecules. The most serious biological damage results when these radioactive emissions fragment or ionize molecules. For example, alpha and beta particles emitted from nuclear decay reactions possess much higher energies than ordinary chemical bond energies. When these particles strike and penetrate matter, they produce ions and molecular fragments that are extremely reactive. The damage this does to biomolecules in living organisms can cause serious malfunctions in normal cell processes, taxing the organism’s repair mechanisms and possibly causing illness or even death (Figure \(\PageIndex{1}\)).
The ability of radiation to damage molecules is analyzed in terms of what is called ionizing power. When a radiation particle interacts with atoms, the interaction can cause the atom to lose electrons and thus become ionized. The greater the likelihood that damage will occur by an interaction is the ionizing power of the radiation. Ionizing radiation could affect either the whole body (somatic damage) and/or eggs and sperm (genetic damage).
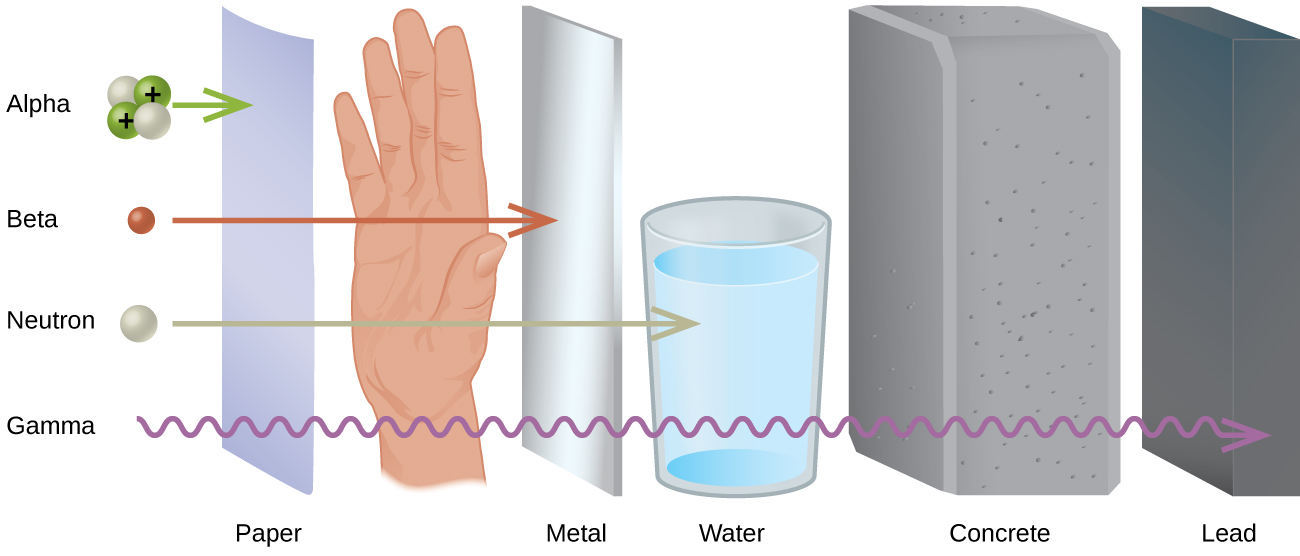
Much of the threat from radiation is involved with the ease or difficulty of protecting oneself from the particles or the gamma ray. How thick of a wall do you need to hide behind to be safe? The ability of each type of radiation to pass through matter is expressed in terms of penetration power. The more material the radiation can pass through, the greater the penetration power and the more dangerous they are. In general, the greater mass present the greater the ionizing power and the lower the penetration power.
When comparing the most common forms of ionizing radiation (alpha, beta, and gamma), alpha particles have the greatest mass. Alpha particles have approximately four times the mass of a proton or neutron and approximately ~8,000 times the mass of a beta particle (Figure \(\PageIndex{1}\)). Because of the large mass of the alpha particle, it has the highest ionizing power and the greatest ability to damage tissue. That same large size of alpha particles, however, makes them less able to penetrate matter. They collide with molecules very quickly when striking matter, add two electrons, and become a harmless helium atom. Alpha particles have the least penetration power and can be stopped by a thick sheet of paper or even a layer of clothes. They are also stopped by the outer layer of dead skin on people. This may seem to remove the threat from alpha particles but only from external sources. In a situation like a nuclear explosion or some sort of nuclear accident where radioactive emitters are spread around in the environment, the emitters can be inhaled or taken in with food or water and once the alpha emitter is inside you, you have no protection at all.
| Particle | Symbol | Mass | Penetrating Power | Ionizing Power | Shielding |
|---|---|---|---|---|---|
| alpha | \(\alpha\) | 4 amu | Very Low | Very High | Paper and Skin |
| beta | \(\beta\) | 1/1837 amu | Intermediate | Intermediate | Aluminum |
| gamma | \(\gamma\) | 0 (energy only) | Very High | Very Low | 2 inches lead |
Beta particles are much smaller than alpha particles, and therefore,they have much less ionizing power (less ability to damage tissue), but their small size gives them much greater penetration power. Most resources say that beta particles can be stopped by a one-quarter inch thick sheet of aluminum. Once again, however, the greatest danger occurs when the beta emitting source gets inside of you.
Gamma rays are not particles but a high energy form of electromagnetic radiation (like x-rays except more powerful). Gamma rays are energy that has no mass or charge. Gamma rays have tremendous penetration power and require several inches of dense material (like lead) to shield them. Gamma rays may pass all the way through a human body without striking anything. They are considered to have the least ionizing power and the greatest penetration power.
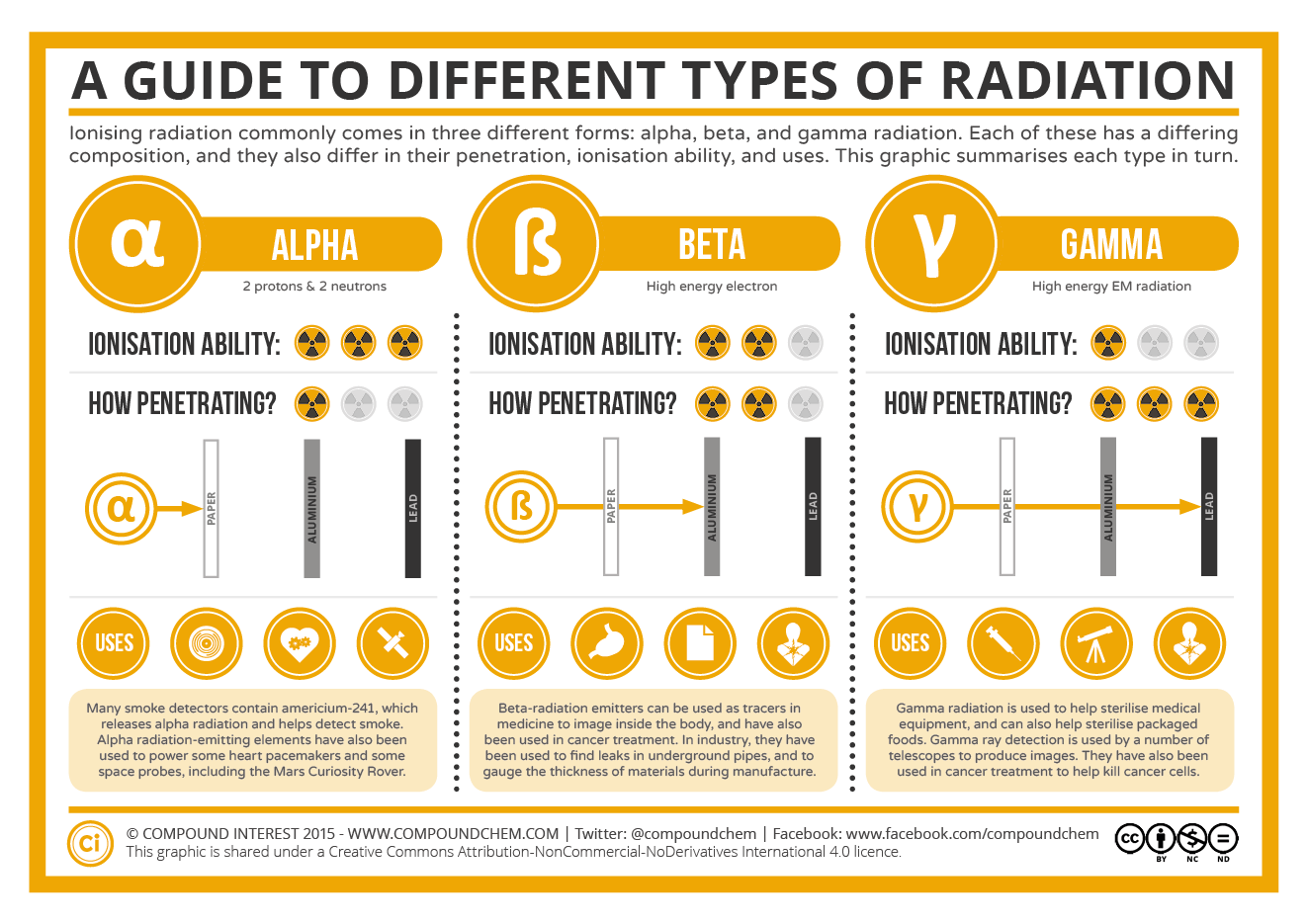
The safest amount of radiation to the human body is zero. It is not possible to be not exposed to ionizing radiation so the next best goal is to be exposed to as little as possible. The two best ways to minimize exposure is to limit time of exposure and to increase distance from the source. The image below summarizes the key concepts of ionization and penetration abilities of alpha, beta, and gamma radiation.
Nonionizing Radiation
There is a large difference in the magnitude of the biological effects of nonionizing radiation (for example, light and microwaves) and ionizing radiation, emissions energetic enough to knock electrons out of molecules, for example, \(α\) and \(β\) particles, \(γ\) rays, X-rays, and high-energy ultraviolet radiation (Figure \(\PageIndex{2}\)).
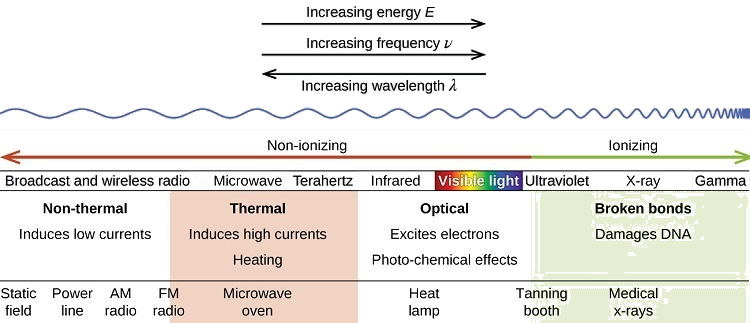
Energy absorbed from nonionizing radiation speeds up the movement of atoms and molecules, which is equivalent to heating the sample. Although biological systems are sensitive to heat (as we might know from touching a hot stove or spending a day at the beach in the sun), a large amount of nonionizing radiation is necessary before dangerous levels are reached. Forms of nonionizing radiation include wave-like radiation shown on the left side of the image. This type of radiation would include the visible spectrum through radio waves.
Everyday Exposure to Radiation
Natural radiation provides the majority of exposure to the average person. Looking at the pie chart below, the largest sources of exposure to radiation is from radon gas (Rn-222). This isotope is an α emitter with a half–life of 3.82 days. Radon is produced through the radioactive decay of U-238, which is found in trace amounts in soil and rocks. In the environment, radon concentrations can vary depending upon a geographical location. Once the soil of a particular region is disturbed, this element can escape and cause serious health issues. Please be aware that radon, and not nuclear reactors, affect more people in the United States.
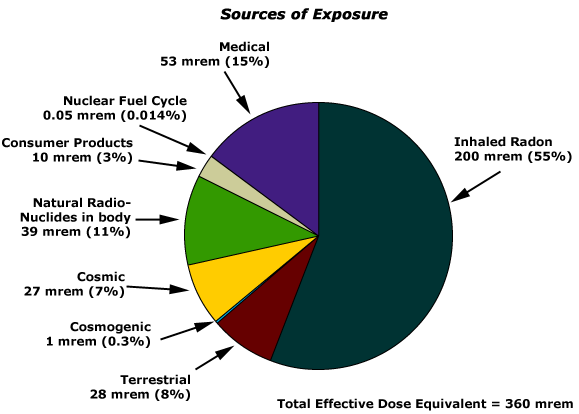
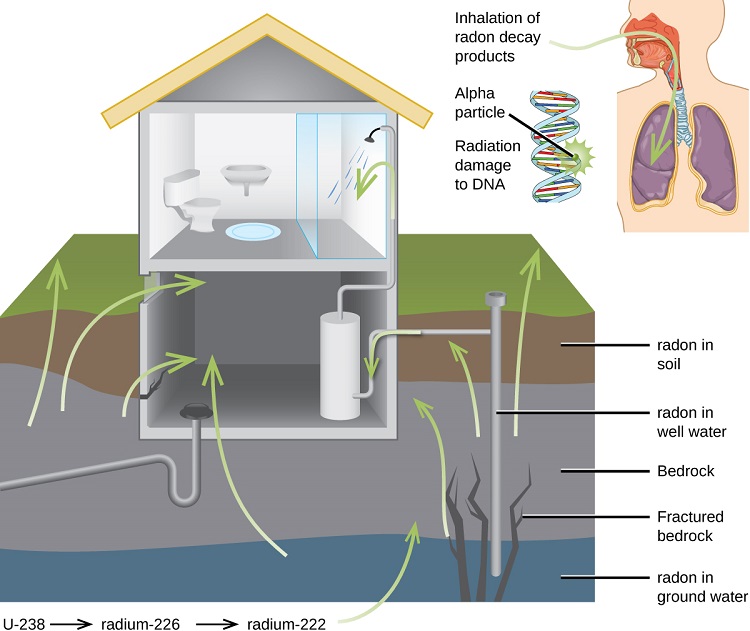
Radon gas escapes from the ground and gradually seeps into homes and other structures above. Since it is about eight times more dense than air, radon gas accumulates in basements and lower floors and slowly diffuses throughout buildings (Figure \(\PageIndex{5}\)). Once airborne, the radon enters the body through inhalation or ingestion. Through alpha emission, Rn-222 decays to produce large particles. These radioactive species travel to the respiratory tract where they will ionize lung tissue. Exposure to radon increases one’s risk of getting cancer (especially lung cancer), and high radon levels can be as bad for health as smoking a carton of cigarettes a day. Radon is the number one cause of lung cancer in nonsmokers and the second leading cause of lung cancer overall. Radon exposure is believed to cause over 20,000 deaths in the US per year.
Radon is found in buildings across the country, with amounts depending on where you live. The average concentration of radon inside houses in the US (1.25 pCi/L) is about three times the levels found in outside air, and about one in six houses have radon levels high enough that remediation efforts to reduce the radon concentration are recommended. The Environmental Protection Agency(EPA) tests homes throughout the United States. This agency classifies their test results into three different zones. Using color-coded keys, an individual can access their probability of being exposed to this deadly isotope. The EPA recommends an individual alter their home if the radon level exceeds 4 pCi/L. Click on this link to see your state and even county's radon level. Red-shaded areas of the EPA map indicate that tested radon levels exceed the 4 pCi/L concentration.
Radon tests can be purchased through the EPA or at most hardware stores. If you are a resident of South Carolina, you can request a free radon test kit through DHEC (Department of Health and Environmental Control). If a consumer finds excessive radon in their home, then alterations to the existing construction can be made. Some of these changes include installing barriers between the soil and the home and placing ventilation on the group floor to release radon outside. For more information regarding these types of alterations, click on this link.
Other sources of radiation in consumer products include smoke detectors, antique watches/clocks, and older ceramics/glass. Smoke detectors emit alpha particles of Am-241 (Americium). Antique watches and clocks use H-3 (tritium), Pm-147 (promethium), or Ra-226 (radium) as a fluorescent light source. Antique ceramics (dating before 1970) could contain U-238 if color is an orangey-red. If antique glassware is yellow or green in color, it could contain U-238 as well. Glassware with this isotope will glow under a black light.
Concrete, fertilizers, kitty litter, and even food (bananas and salt substitutes) can contain trace amounts of different types of radioactivity. K-40 (potassium) is present in all of these substances.
Need More Practice?
- Turn to Section 5.E of this OER and answer questions #3, #6, #9, and #10.
References
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Emma Gibney (Furman University)


