5.3: Types of Radiation
- Page ID
- 85510
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
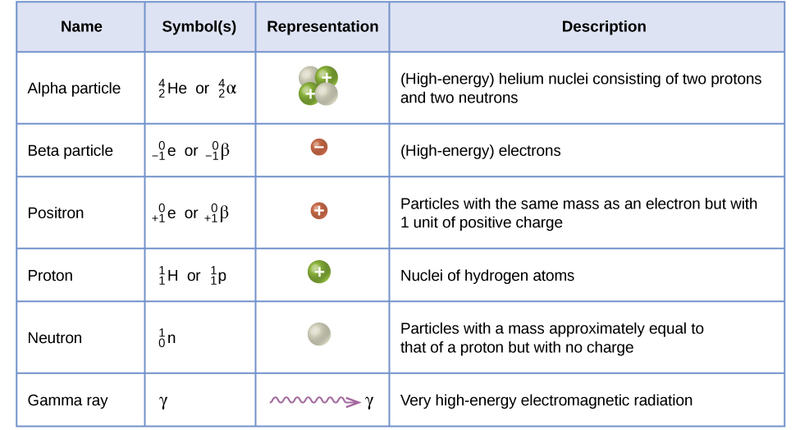
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Become familiar with A/Z formats for alpha, beta, positron, neutron, gamma, and the three isotopes of hydrogen.
- Express the changes in the atomic number and mass number of radioactive nuclei when a particle or ray is emitted.
- Convert symbol-mass format of elements to A/Z format and then balance a nuclear reaction.
- Generate a decay reaction if provided with a symbol-mass format of an element.
Many nuclei are radioactive; that is, they decompose by emitting particles or rays and in doing so, become a different nucleus. In our studies up to this point, atoms of one element were unable to change into different elements. That is because in all other types of changes we have talked about only the electrons were changing. In these changes, the nucleus, which contains the protons that dictate which element an atom is, is changing. All nuclei with 84 or more protons are radioactive and elements with less than 84 protons have both stable and unstable isotopes. All of these elements can go through nuclear changes and turn into different elements.
In natural radioactive decay, three common emissions occur. When these emissions were originally observed, scientists were unable to identify them as some already known particles and so named them
- alpha particles (\(\alpha \)),
- beta particles, \(\left( \beta \right)\), and
- gamma rays \(\left( \gamma \right)\)
using the first three letters of the Greek alphabet. At some later time, alpha particles were identified as helium-4 nuclei, beta particles were identified as electrons, and gamma rays as a form of electromagnetic radiation like x-rays except much higher in energy and even more dangerous to living systems.
Alpha Decay
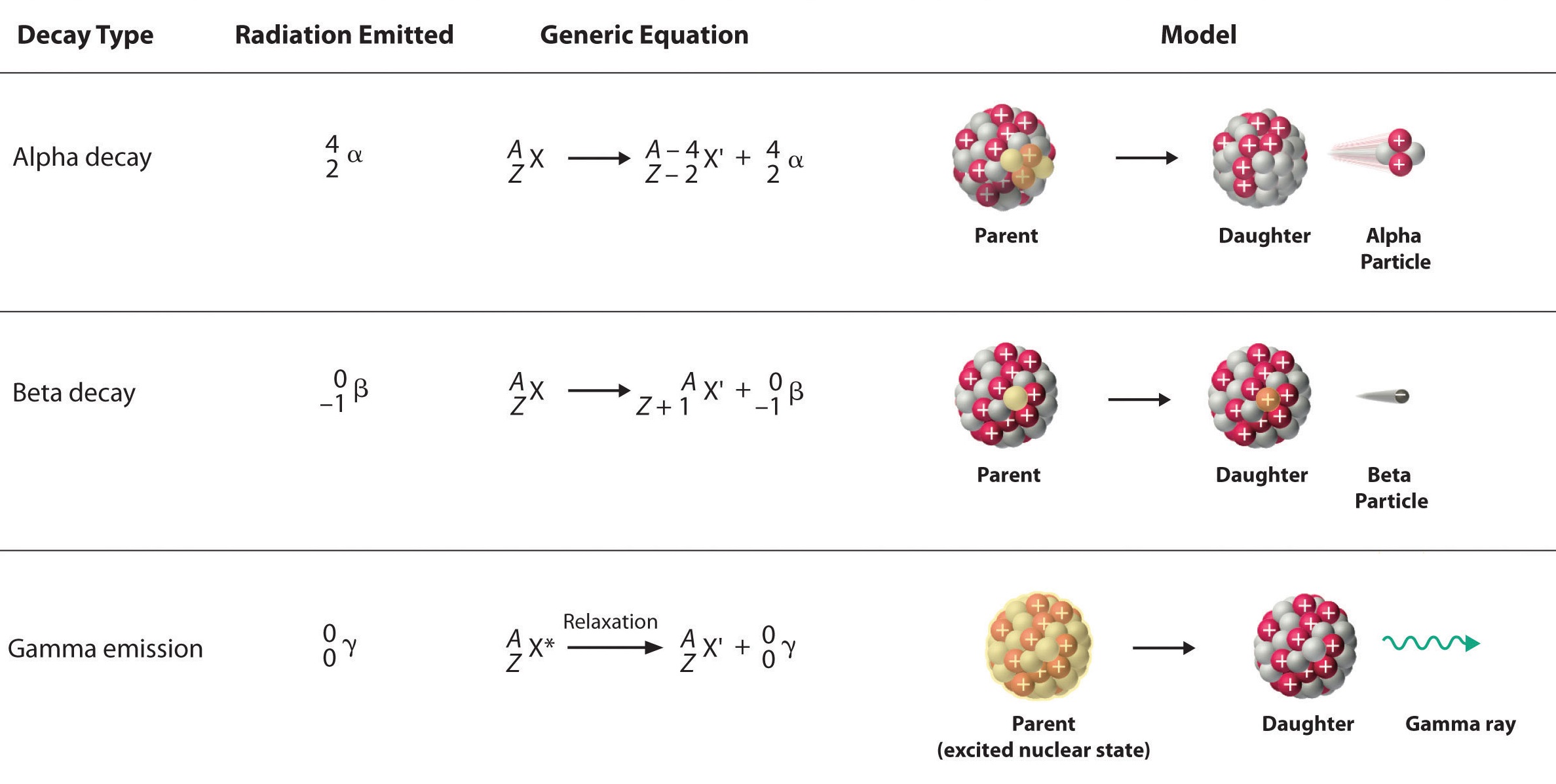
The nuclear disintegration process that emits alpha particles is called alpha decay. An example of a nucleus that undergoes alpha decay is uranium-238. The alpha decay of \(\ce{U}\)-238 is
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{90}^{234}Th} \label{alpha1} \]
In this nuclear change, the uranium atom \(\left( \ce{_{92}^{238}U} \right)\) transmuted into an atom of thorium \(\left( \ce{_{90}^{234}Th} \right)\) and, in the process, gave off an alpha particle. Look at the symbol for the alpha particle: \(\ce{_2^4He}\). Where does an alpha particle get this symbol? The bottom number in a nuclear symbol is the number of protons. That means that the alpha particle has two protons in it which were lost by the uranium atom. The two protons also have a charge of \(+2\). The top number, 4, is the mass number or the total of the protons and neutrons in the particle. Because it has 2 protons, and a total of 4 protons and neutrons, alpha particles must also have two neutrons. Alpha particles always have this same composition: two protons and two neutrons.
Another alpha particle producer is thorium-230.
\[\ce{_{90}^{230}Th} \rightarrow \ce{_2^4He} + \ce{_{88}^{226}Ra} \label{alpha2} \]
Alpha decays occur with radioactive isotopes of radium, radon, uranium, and thorium.
Beta Decay
Another common decay process is beta particle emission, or beta decay. A beta particle is simply a high energy electron that is emitted from the nucleus. It may occur to you that we have a logically difficult situation here. Nuclei do not contain electrons and yet during beta decay, an electron is emitted from a nucleus. At the same time that the electron is being ejected from the nucleus, a neutron is becoming a proton. It is tempting to picture this as a neutron breaking into two pieces with the pieces being a proton and an electron. That would be convenient for simplicity, but unfortunately that is not what happens; more about this at the end of this section. For convenience sake, though, we will treat beta decay as a neutron splitting into a proton and an electron. The proton stays in the nucleus, increasing the atomic number of the atom by one. The electron is ejected from the nucleus and is the particle of radiation called beta.
To insert an electron into a nuclear equation and have the numbers add up properly, an atomic number and a mass number had to be assigned to an electron. The mass number assigned to an electron is zero (0) which is reasonable since the mass number is the number of protons plus neutrons and an electron contains no protons and no neutrons. The atomic number assigned to an electron is negative one (-1), because that allows a nuclear equation containing an electron to balance atomic numbers. Therefore, the nuclear symbol representing an electron (beta particle) is
\(\ce{_{-1}^0e}\) or \(\ce{_{-1}^0\beta} \label{beta1}\)
Thorium-234 is a nucleus that undergoes beta decay. Here is the nuclear equation for this beta decay.
\[\ce{_{90}^{234}Th} \rightarrow \ce{_{-1}^0e} + \ce{_{91}^{234}Pa} \label{beta2} \]
Beta decays are common with Sr-90, C-14, H-3 and S-35.
Gamma Radiation
Frequently, gamma ray production accompanies nuclear reactions of all types. In the alpha decay of \(\ce{U}\)-238, two gamma rays of different energies are emitted in addition to the alpha particle.
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{90}^{234}Th} + 2 \ce{_0^0\gamma} \nonumber \]
Virtually all of the nuclear reactions in this chapter also emit gamma rays, but for simplicity the gamma rays are generally not shown. Nuclear reactions produce a great deal more energy than chemical reactions. Chemical reactions release the difference between the chemical bond energy of the reactants and products, and the energies released have an order of magnitude of \(1 \times 10^3 \: \text{kJ/mol}\). Nuclear reactions release some of the binding energy and may convert tiny amounts of matter into energy. The energy released in a nuclear reaction has an order of magnitude of \(1 \times 10^{18} \: \text{kJ/mol}\). That means that nuclear changes involve almost a million times more energy per atom than chemical changes!
Common gamma emitters would include I-131, Cs-137, Co-60, and Tc-99.
Virtually all of the nuclear reactions in this chapter also emit gamma rays, but for simplicity the gamma rays are generally not shown.
The essential features of each reaction are shown in Figure \(\PageIndex{2}\).
"Nuclear Accounting"
When writing nuclear equations, there are some general rules that will help you:
- The sum of the mass numbers (top numbers) on the reactant side equal the sum of the mass numbers on the product side.
- The atomic numbers (bottom numbers) on the two sides of the reaction will also be equal.
In the alpha decay of \(\ce{^{238}U}\) (Equation \(\ref{alpha1}\)), both atomic and mass numbers are conserved:
- mass number: \(238 = 4 + 234\)
- atomic number: \(92 = 2 + 90\)
Confirm that this equation is correctly balanced by adding up the reactants' and products' atomic and mass numbers. Also, note that because this was an alpha reaction, one of the products is the alpha particle, \(\ce{_2^4He}\).
Note that both the mass numbers and the atomic numbers add up properly for the beta decay of Thorium-234 (Equation \(\ref{beta2}\)):
- mass number: \(234 = 0 + 234\)
- atomic number: \(90 = -1 + 91\)
The mass numbers of the original nucleus and the new nucleus are the same because a neutron has been lost, but a proton has been gained and so the sum of protons plus neutrons remains the same. The atomic number in the process has been increased by one since the new nucleus has one more proton than the original nucleus. In this beta decay, a thorium-234 nucleus has one more proton than the original nucleus. In this beta decay, a thorium-234 nucleus has become a protactinium-234 nucleus. Protactinium-234 is also a beta emitter and produces uranium-234.
\[\ce{_{91}^{234}Pa} \rightarrow \ce{_{-1}^0e} + \ce{_{92}^{234}U} \label{nuke1} \]
Once again, the atomic number increases by one and the mass number remains the same; confirm that the equation is correctly balanced. Before starting the problems, below preview this video of your instructor balancing nuclear reactions.
Complete the following nuclear reaction by filling in the missing particle.
\[\ce{_{86}^{210}Rn -> _2^4He \,+ \,?} \nonumber \]
Solution
This reaction is an alpha decay. We can solve this problem one of two ways:
Solution 1: When an atom gives off an alpha particle, its atomic number drops by 2 and its mass number drops by 4 leaving: \(\ce{_{84}^{206}Po}\). We know the symbol is \(\ce{Po}\), for polonium, because this is the element with 84 protons on the periodic table.
Solution 2: Remember that the mass numbers on each side must total up to the same amount. The same is true of the atomic numbers.
- Mass numbers: \(210 = 4 + ?\)
- Atomic numbers: \(86 = 2 + ?\)
We are left with \(\ce{_{84}^{206}Po}\)
Write each of the following nuclear reactions.
- Carbon-14, used in carbon dating, decays by beta emission.
- Uranium-238 decays by alpha emission.
Solution
a) Beta particles have the symbol \(\ce{_{-1}^0e}\). Emitting a beta particle causes the atomic number to increase by 1 and the mass number to not change. We get atomic numbers and symbols for elements using our periodic table. We are left with the following reaction:
\[\ce{_6^{14}C} \rightarrow \ce{_{-1}^0e} + \ce{_7^{14}N} \nonumber \]
b) Alpha particles have the symbol \(\ce{_2^4He}\). Emitting an alpha particle causes the atomic number to decrease by 2 and the mass number to decrease by 4. We are left with:
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{90}^{234}Th} \nonumber \]
Decay Series
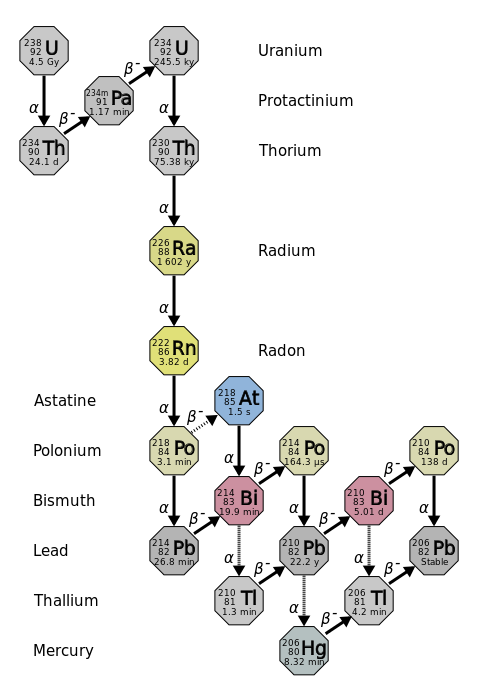
The decay of a radioactive nucleus is a move toward becoming stable. Often, a radioactive nucleus cannot reach a stable state through a single decay. In such cases, a series of decays will occur until a stable nucleus is formed. The decay of \(\ce{U}\)-238 is an example of this. The \(\ce{U}\)-238 decay series starts with \(\ce{U}\)-238 and goes through fourteen separate decays to finally reach a stable nucleus, \(\ce{Pb}\)-206 (Figure \(\PageIndex{3}\)). There are similar decay series for \(\ce{U}\)-235 and \(\ce{Th}\)-232. The \(\ce{U}\)-235 series ends with \(\ce{Pb}\)-207 and the \(\ce{Th}\)-232 series ends with \(\ce{Pb}\)-208.
.svg.png?revision=1&size=bestfit&width=460&height=648)
Several of the radioactive nuclei that are found in nature are present there because they are produced in one of the radioactive decay series. For example, there may have been radon on the earth at the time of its formation, but that original radon would have all decayed by this time. The radon that is present now is present because it was formed in a decay series (mostly by U-238).
Need More Practice?
- Turn to Section 5.E of this OER and work problems #1 and #2.


