2.2: Dimensional Analysis
- Page ID
- 85139
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Convert metric and English based units.
- Report converted values in correct scientific notation with the corresponding units.
- Master using your calculator.
Conversion Factors
Recall your metric table (one of two that needs to be committed to memory) while working on these types of problems.
Using negative exponents (see above) is the traditional way to memorize the metric system. Many students who have been taught outside of the United States approach the metric system this way. For their conversion factors, students who have been taught the metric system in the United States tend to use positive exponents. Either method is fine, just pick one way and use it consistently.
Two different ways to memorize the metric systems (be sure to know all bolded conversion factors listed in Section 2.1)
- 1 gigabase = 1 x109 base
- 1 megabase = 1x106 base
- 1 kilobase = 1x103 base
- 1 decibase = 1x10-1 base or 1x101 decibase = 1 base
- 1 centibase = 1x10-2 base or 1x102 centibase = 1 base
- 1 millibase = 1x10-3 base or 1x103 millibase = 1 base
- 1 microbase = 1x10-6 base or 1x106 microbase = 1 base
- 1 nanobase = 1x10-9 base or 1x109 nanobase = 1 base
- 1 picobase = 1x10-12 base or 1x1012 picobase = 1 base
Note: A base unit can be gram, liter, or meter
We have an expression,
\[\dfrac{3\,ft}{1\,yd} =1 \label{1} \]
Equation \ref{1} is a strange way to write 1, but it makes sense: 3 ft equal 1 yd, so the quantities in the numerator and denominator are the same quantity, just expressed with different units. The expression in Equation \ref{1} is called a conversion factor and it is used to formally change the unit of a quantity into another unit. (The process of converting units in such a formal fashion is sometimes called dimensional analysis or the factor label method.)
To see how this happens, let us start with the original quantity: \(4\, yd\) and let us multiply this quantity by 1. Rather than multiplying by just 1, let us write 1 as
\[ 1= \dfrac{3 \, ft}{1 \, yd} \nonumber \]
When you multiply anything by 1, you don’t change the value of the quantity.
\[\begin{align*} 4 \, yd &= 4 \, yd \times 1 \\[4pt] &= 4 \, yd \times \dfrac{3 \, ft}{1 \, yd} \end{align*} \]
The 4 yd term can be thought of as yd/1; that is, it can be thought of as a fraction with 1 in the denominator. We are essentially multiplying fractions. If the same thing appears in the numerator and denominator of a fraction, they cancel. In this case, what cancels is the unit yard:
\[4 \, \cancel{yd}\times \dfrac{3 \, ft}{1 \, \cancel{yd}} \nonumber \]
That is all that we can cancel. Now, multiply and divide all the numbers to get the final answer:
\[\dfrac{4\times 3 \, ft}{1}= \dfrac{12 \, ft}{1}= 12 \, ft \nonumber \]
Again, we get an answer of 12 ft, just as we did originally. But in this case, we used a more formal procedure that is applicable to a variety of problems.
How many millimeters are in 14.66 m? To answer this, we need to construct a conversion factor between millimeters and meters and apply it correctly to the original quantity. One method would entail using the conversion factor of \(1\, mm = 1 \times 10^{-3}\,m\)
\[\begin{align} 14.66\,\cancel{m}\times \dfrac{1\,mm}{10^{-3}\,\cancel{m}} &= 14660\,mm \\[5pt] &= 1.46\times 10^{4}\,mm \end{align} \nonumber \]
Which conversion factor do we use? The answer is based on what unit you want to get rid of in your initial quantity. The original unit of our quantity is meters, which we want to convert to millimeters. Because the original unit is assumed to be in the numerator, to get rid of it, we want the meter unit in the denominator; then they will cancel. Using the positive metric exponents, we have an alternative method to calculate the same answer as shown previously. Canceling units and performing the mathematics, we get:
\[\begin{align} 14.66\, \cancel{m} \times \dfrac{1000\,mm}{1\,\cancel{m}} &= 14660\,mm \\[5pt] &= 1.46\times 10^{4}\,mm \end{align} \nonumber \]
Note how \(m\) cancels, leaving \(mm\), which is the unit of interest.
The ability to construct and apply proper conversion factors is a very powerful mathematical technique in chemistry. You need to master this technique if you are going to be successful in this and future science courses.
Check out this fabulous light board of me modeling problem solving using this method. For Furman University students, this video is displayed in moodle and box.
Warning: my last answer is off by a factor of 10. It should be 4.8x10-2kg.
- Convert 35.9 kL to liters.
- Convert 555 nm to meters.
Solution
- We will use the fact that 1 kL = 1,000 L. Of the two conversion factors that can be defined, the one that will work is 1000L/ 1kL. Applying this conversion factor, we get:
\[35.9\, \cancel{kL} \times \dfrac{1000\,L}{1\, \cancel{kL}}= 35,900\,L \nonumber \]
- We will use the fact that 1 nm = 1/1,000,000,000 m, which we will rewrite as 1,000,000,000 nm = 1 m, or 109 nm = 1 m. Of the two possible conversion factors, the appropriate one has the nm unit in the denominator:
\[\dfrac{1\,m}{10^{9}\,m} \nonumber \]
Applying this conversion factor, we get
\[\begin{align*}555\,\cancel{nm} \times \dfrac{1\,m}{10^{9}\,\cancel{nm}} &= 0.000000555\,m \\[5pt] &= 5.55\times 10^{-7}\,m \end{align*} \]
In the final step, we expressed the answer in scientific notation.
- Convert 67.08 μL to liters.
- Convert 56.8 m to kilometers.
- Answer a
-
6.708 × 10−5 L
- Answer b
-
5.68 × 10−2 km
How many milliliters are in 607.8 kL?
- Answer
- This problem involves multiple steps that require going through the metric base of liters. Think of a pathway of a conversion before setting up these types of problems. The suggested pathway would be mL ⇒ L ⇒ kL
\[ \begin{align*} 607.8 \,kL \times \dfrac{1000\,L}{1\,kL} &= 6.08\times 10^{5}\,L \nonumber \\[5pt] &= 6.08\times 10^5 \, L \dfrac{1000\,mL}{1\,L} \\[5pt] &= 608000000\,mL \\[5pt] &= 6.08\times 10^{8}\,mL \nonumber \end{align*} \]
Kathleen Hsu Barron (Furman University, Class of 2011) is exploring Switzerland with her husband Patrick Barron (Furman University, Class of 2011). The sign she is pointing to is a typical speed in km/hr. Convert this distance to miles/hr and feet/hr.

- Answer
-
Again, this problem is a multi-step problem. There are multiple ways that one could proceed to solve this problem. With the conversion factors in this course, the suggested round would be miles ⇒ kilometers ⇒ meters ⇒ centimeters ⇒ inches ⇒ feet. Another method could utilize miles ⇒ feet.
18.6 miles/hr, 9.8 x 104 ft/hr
In this course, we will not use significant figures. You will need to read the directions on your assignment, quiz, or test and round accordingly.
The skill of converting units can be helpful in many aspects of your life. Measuring medications can be done precisely by using milliliters instead of teaspoons (recall 1 tsp = 5.0 mL). Of course, a pharmacist would provide you with the appropriate syringe if this was needed. Sizes of syringes can vary from 1.0 to 10.0 mL for most at-home applications.
Marathons are measured using the kilometer unit. If you were raised outside the United States, you would be quite familiar with how long a kilometer truly is. To appreciate this distance, most Americans need to convert this to mileage by dividing the distance by 1.61 km.
When traveling abroad, keep in mind the majority of countries (except Myanmar and Liberia) commonly use the metric system in day to day living. Understanding how to convert Metric to English units will help you to appreciate the measurements used by a different society.
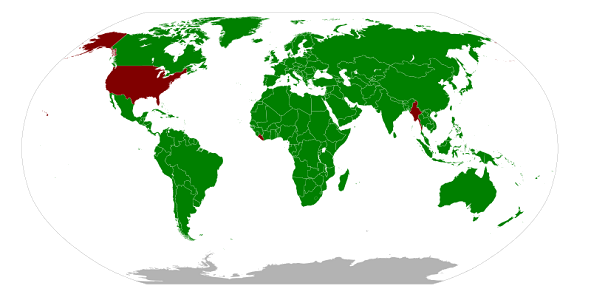
Why has the United States not adopted the metric system as a country? Watch this video and see if you can derive some appropriate hypotheses.
Need More Practice?
- Turn to Section 2.E of this OER and work problems #1, #3, #5, #6, and #8.
Contributors and Attributions
- Hayden Cox (Furman University)
- Makena Song (Furman University)


