1.1: What is Chemistry?
- Page ID
- 85131
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To recognize the breadth, depth, and scope of chemistry.
- Define chemistry in relation to other sciences.
- Identify the main disciplines of chemistry.
- Match a scientific project with the correct type of chemist.
Chemistry is the study of matter—what it consists of, what its properties are, and how it changes. Being able to describe the ingredients in a cake and how they change when the cake is baked is called chemistry. Matter is anything that has mass and takes up space—that is, anything that is physically real. Some things are easily identified as matter—this book, for example. Others are not so obvious. Because we move so easily through the air, we sometimes forget that it, too, is matter.
Chemistry is one branch of science. Science is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. Because the physical universe is so vast, there are many different branches of science (Figure \(\PageIndex{1}\)). Thus, chemistry is the study of matter, biology is the study of living things, and geology is the study of rocks and the earth. Mathematics is the language of science, and we will use it to communicate some of the ideas of chemistry.

Although we divide science into different fields, there is much overlap among them. For example, some biologists and chemists work in both fields so much that their work is called biochemistry. Similarly, geology and chemistry overlap in the field called geochemistry. Figure \(\PageIndex{1}\) shows how many of the individual fields of science are related.
There are many other fields of science, in addition to the ones (biology, medicine, etc.) listed
As our understanding of the universe has changed over time, so has the practice of science. Chemistry in its modern form, based on principles that we consider valid today, was developed in the 1600s and 1700s. Before that, the study of matter was known as alchemy and was practiced mainly in China, Arabia, Egypt, and Europe.
Alchemy was a somewhat mystical and secretive approach to learning how to manipulate matter. Practitioners, called alchemists, thought that all matter was composed of different proportions of the four basic elements—fire, water, earth, and air—and believed that if you changed the relative proportions of these elements in a substance, you could change the substance. The long-standing attempts to “transmute” common metals into gold represented one goal of alchemy. Alchemy’s other major goal was to synthesize the philosopher’s stone, a material that could impart long life—even immortality. Alchemists used symbols to represent substances, some of which are shown in the accompanying figure. This was not done to better communicate ideas, as chemists do today, but to maintain the secrecy of alchemical knowledge, keeping others from sharing in it.
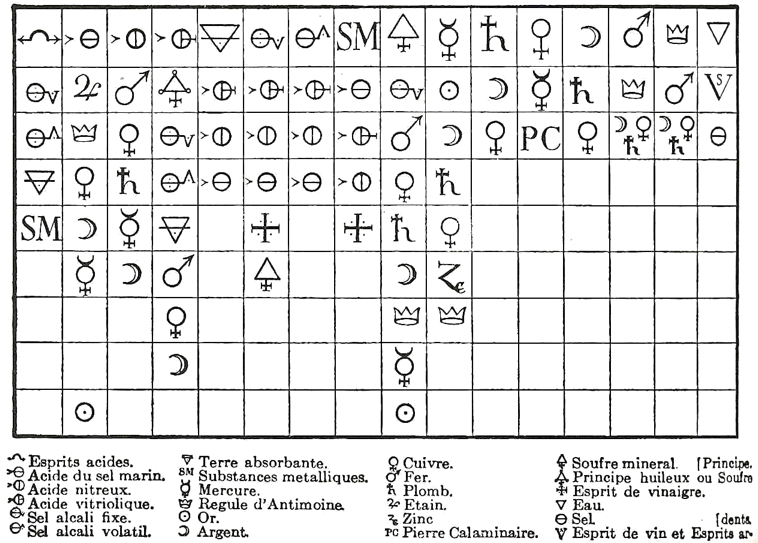
In spite of this secrecy, in its time alchemy was respected as a serious, scholarly endeavor. Isaac Newton, the great mathematician and physicist, was also an alchemist.
While watching the video below and answer the following questions.
Questions
- What was the chief goal of an alchemist according to the video?
- What could the philosopher’s stone do to urine?
- Is Alchemy a true science?
- When urine is boiled down to a white paste, what is the name and symbol for the element that was obtained?
- List some properties of this element that were discussed in the video.
- Did wealthy people produce more of this element than poorer people?
- What types of applications (applied science) did this element lead us to?
- Instead of collecting urine, how can one collect higher concentrations of this element?
- The video discussed phosphoric acid (formula: H3PO4). Name all the elements in this compound.
- What were the mentioned applications of phosphoric acid?
- What are some of the organic and biochemical applications of element 13?
Areas of Chemistry
The study of modern chemistry has many branches, but can generally be broken down into five main disciplines, or areas of study:
- Physical chemistry: Physical chemistry is the study of macroscopic properties, atomic properties, and phenomena in chemical systems. A physical chemist may study such things as the rates of chemical reactions, the energy transfers that occur in reactions, or the physical structure of materials at the molecular level.
- Organic chemistry: Organic chemistry is the study of chemicals containing carbon with hydrogen. Carbon is one of the most abundant elements on Earth and is capable of forming a tremendously vast number of chemicals (over twenty million so far). Most of the chemicals found in all living organisms are based on carbon.
- Inorganic chemistry: Inorganic chemistry is the study of chemicals that do not, in general, contain carbon. Inorganic chemicals are commonly found in rocks and minerals. One current important area of inorganic chemistry deals with the design and properties of materials involved in energy and information technology.
- Analytical chemistry: Analytical chemistry is the study of the composition of matter. It focuses on separating, identifying, and quantifying chemicals in samples of matter. An analytical chemist may use complex instruments to analyze an unknown material in order to determine its various components.
- Biochemistry: Biochemistry is the study of chemical processes that occur in living things. Research may cover basic cellular processes up to understanding disease states so better treatments can be developed.

In practice, chemical research is often not limited to just one of the five major disciplines. A particular chemist may use biochemistry to isolate a particular chemical found in the human body such as hemoglobin, the oxygen-carrying component of red blood cells. He or she may then proceed to analyze the hemoglobin using methods that would pertain to the areas of physical or analytical chemistry. Many chemists specialize in areas that are combinations of the main disciplines, such as bioorganic chemistry or physical organic chemistry.
The American Chemical Society (ACS) has designed a series of videos illustrating the different fields that a chemist could pursue. Please watch this 2 minute and 23-second video and answer the questions below:
- Which type of chemistry does Dr. Jacobs explore (look at the five types of chemists listed above).
- How do Dr. Jacobs and her research associates apply their chemistry to a real-world problem?
- What types of professionals does Dr. Jacobs collaborate with?
- Which are more difficult to characterize and why: proteins or small molecules?
Summary
- Chemistry is the study of matter and the changes it undergoes and considers both macroscopic and microscopic information.
- Matter is anything that has mass and occupies space.
- The five main disciplines of chemistry are physical chemistry, organic chemistry, Inorganic chemistry, analytical chemistry, and biochemistry.
- Many civilizations contributed to the growth of chemistry. A lot of early chemical research focused on practical uses. Basic chemistry theories were developed during the nineteenth century. New materials and batteries are a few of the products of modern chemistry.
Contributors and Attributions
- Hayden Cox (Furman University)


