3.6: Stratospheric Ozone- Earth's Vital Shield
- Page ID
- 466591
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the depletion of the ozone layer.
- Explain how chlorine and bromine atoms react with ozone which leads to the depletion of the ozone layer.
The earth's stratospheric ozone layer plays a critical role in absorbing ultraviolet radiation emitted by the sun. In the last thirty years, it has been discovered that stratospheric ozone is depleting due to anthropogenic pollutants. There are several chemical reactions that can deplete stratospheric ozone; however, some of the most significant of these involve the catalytic destruction of ozone by halogen radicals such as chlorine and bromine.
Introduction
The atmosphere of the Earth is divided into five layers. In order of closest and thinnest to farthest and thickest the layers are listed as follows: troposphere, stratosphere, mesosphere, thermosphere and exosphere. The majority of the ozone in the atmosphere resides in the stratosphere, which extends from six miles above the Earth’s surface to 31 miles. Humans rely heavily on the absorption of ultraviolet B rays by the ozone layer because UV-B radiation causes skin cancer and can lead to genetic damage. The ozone layer has historically protected the Earth from harmful UV rays, although in recent decades this protection has diminished due to stratospheric ozone depletion.
Ozone depletion is largely a result of man-made substances. Humans have introduced gases and chemicals into the atmosphere that have rapidly depleted the ozone layer in the last century. This depletion makes humans more vulnerable to the UV-B rays which are known to cause skin cancer as well as other genetic deformities. The possibility of ozone depletion was first introduced by scientists in the late 1960's as dreams of supersonic transport began to become a reality. Scientists had long been aware that nitric oxide (NO) can catalytically react with ozone (\(O_3\)) to produce \(O_2\) molecules; however, \(NO\) molecules produced at ground level have a half-life far too short to make it into the stratosphere. It was not until the advent of commercial supersonic jets (which fly in the stratosphere and at an altitude much higher than conventional jets) that the potential for \(NO\) to react with stratospheric ozone became a possibility. The threat of ozone depletion from commercial supersonic transport was so great that it is often cited as the main reason why the US federal government pulled support for its development in 1971. Fear of ozone depletion was abated until 1974 when Sherwood Rowland and Mario Molina discovered that chlorofluorocarbons could be photolyzed by high-energy photons in the stratosphere. They discovered that this process could release chlorine radicals that would catalytically react with \(O_3\) and destroy the molecule. This process is called the Rowland-Molina theory of \(O_3\) depletion.
The Chapman Cycle
The ozone (\(O_3\)) located in the stratosphere is in a constant cycle with oxygen molecules (\(O_2\)) and their interaction with ultraviolet rays. This process is considered a cycle because of its constant conversion between different molecules of oxygen. The ozone layer is created when ultraviolet rays react with oxygen molecules (O2) to create ozone (O3) and atomic oxygen (O). This process is called the Chapman cycle.
Step 1: An oxygen molecule is photolyzed by solar radiation, creating two oxygen radicals:
\[ h\nu + O_2 \rightarrow 2O^. \nonumber \]
Step 2: Oxygen radicals then react with molecular oxygen to produce ozone:
\[O_2 + O^. \rightarrow O_3 \nonumber \]
Step 3: Ozone then reacts with an additional oxygen radical to form molecular oxygen:
\[O_3 + O^. \rightarrow 2O_2 \nonumber \]
Step 4: Ozone can also be recycled into molecular oxygen by reacting with a photon:
\[O_3 + h\nu \rightarrow O_2 + O^. \nonumber \]
It is important to keep in mind that ozone is constantly being created and destroyed by the Chapman cycle and that these reactions are natural processes, which have been taking place for millions of years. Because of this, the thickness of the ozone layer at any particular time can vary greatly. It is also important to know that O2 is constantly being introduced into the atmosphere through photosynthesis, so the ozone layer has the capability of regenerating itself.
Chemistry of Ozone Depletion
Chlorofluorocarbons (CFCs), made up of chlorine, fluorine, and carbon atoms, are extremely stable. This extreme stability allows CFC's to slowly make their way from the troposphere into the stratosphere (most other less-stable molecules decompose before they can cross into the stratosphere from the troposphere). This prolonged life in the atmosphere allows them to reach great altitudes where photons are more energetic. When the CFC's come into contact with these high-energy photons, their individual components are freed from the whole creating highly reactive free radicals. The following reaction displays how Cl atoms have an ozone-destroying cycle:
\[Cl + O_3 \rightarrow ClO + O_2 \tag{step 1} \]
\[ClO + O^. \rightarrow Cl + O_2 \tag{step 2} \]
\[O_3 + O^. \rightarrow 2O_2 \tag{Overall reaction} \]
Chlorine is able to destroy so much of the ozone because it acts as a catalyst, meaning it is regenerated during this process. Chlorine initiates the breakdown of ozone and combines with a freed oxygen atom to create two oxygen molecules. After each reaction, chlorine begins the destructive cycle again with another ozone molecule. One chlorine atom can thereby destroy thousands of ozone molecules. Because ozone molecules are being broken down they are unable to absorb any ultraviolet light so we experience more intense UV radiation at the Earth's surface.
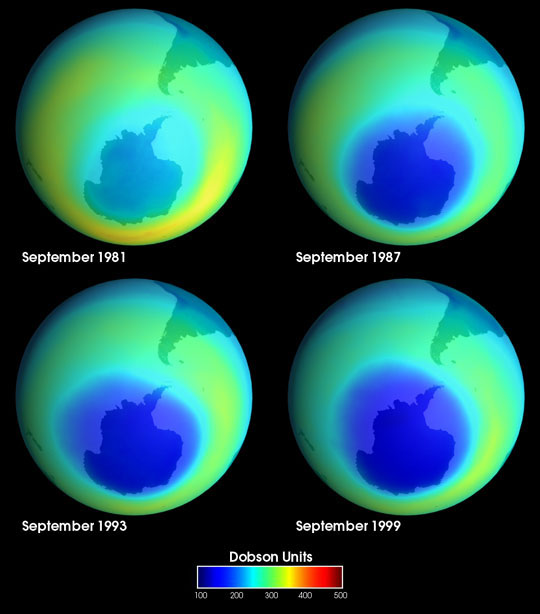
From 1985 to 1988, researchers studying atmospheric properties over the south pole continually noticed significantly reduced concentrations of ozone directly over the continent of Antarctica. For three years it was assumed that the ozone data was incorrect and was due to some instrument malfunction. In 1988, researchers finally realized an instrument malfunction had not occurred and the data was correct. They concluded that an enormous hole in the ozone layer had indeed developed over Antarctica. Examination of NASA satellite data later showed that the hole had begun to develop in the mid-1970's.
The ozone hole over Antarctica is formed by a slew of unique atmospheric conditions over the continent that combine to create an ideal environment for ozone destruction.
- Because Antarctica is surrounded by water, winds over the continent blow in a unique clockwise direction creating a so-called "polar vortex" that effectively contains a single static air mass over the continent. As a result, air over Antarctica does not mix with air in the rest of the Earth's atmosphere.
- Antarctica has the coldest winter temperatures on earth, often reaching -110 °F. These chilling temperatures result in the formation of polar stratospheric clouds (PSC's) which are a conglomeration of frozen H2O and HNO3. Due to their extremely cold temperatures, PSC's form an electrostatic attraction with CFC molecules as well as other halogenated compounds
As spring comes to Antarctica, the PSC's melt in the stratosphere and release all of the halogenated compounds that were previously absorbed into the cloud. In the antarctic summer, high-energy photons are able to photolyze the halogenated compounds, freeing halogen radicals that then catalytically destroy O3. Because Antarctica is constantly surrounded by a polar vortex, radical halogens are not able to be diluted over the entire globe. The ozone hole develops as a result of this process.
Recent research suggests that the strength of the polar vortex from any given year is directly correlated to the size of the ozone hole. In years with a strong polar vortex, the ozone hole is seen to expand in diameter, whereas in years with a weaker polar vortex, the ozone hole is noted to shrink
Ozone Depleting Substances
The following substances are listed as ozone-depleting substances under Title VI of the United States Clean Air Act:
| Substance | Ozone- depletion potential |
|---|---|
| chlorofluorocarbon-11 (CFC–11) | 1.0 |
| chlorofluorocarbon-12 (CFC–12) | 1.0 |
| chlorofluorocarbon-13 (CFC–13) | 1.0 |
| chlorofluorocarbon-111 (CFC–111) | 1.0 |
| chlorofluorocarbon-112 (CFC–112) | 1.0 |
| chlorofluorocarbon-113 (CFC–113) | 0.8 |
| chlorofluorocarbon-114 (CFC–114) | 1.0 |
| chlorofluorocarbon-115 (CFC–115) | 0.6 |
| chlorofluorocarbon-211 (CFC–211) | 1.0 |
| chlorofluorocarbon-212 (CFC–212) | 1.0 |
| chlorofluorocarbon-213 (CFC–213) | 1.0 |
| chlorofluorocarbon-214 (CFC–214) | 1.0 |
| chlorofluorocarbon-215 (CFC–215) | 1.0 |
| chlorofluorocarbon-216 (CFC–216) | 1.0 |
| chlorofluorocarbon-217 (CFC–217) | 1.0 |
| halon-1211 | 3.0 |
| halon-1301 | 10.0 |
| halon-2402 | 6.0 |
| carbon tetrachloride | 1.1 |
| methyl chloroform | 0.1 |
| hydrochlorofluorocarbon-22 (HCFC–22) | 0.05 |
| hydrochlorofluorocarbon-123 (HCFC–123) | 0.02 |
| hydrochlorofluorocarbon-124 (HCFC–124) | 0.02 |
| hydrochlorofluorocarbon-141(b) (HCFC–141(b)) | 0.1 |
| hydrochlorofluorocarbon-142(b) (HCFC–142(b)) | 0.06 |
Montreal Protocol
International policy efforts to restrict production of ozone-depleting CFCs culminated in the 1987 treaty known as the Montreal Protocol in which signing nations agreed to cut CFC production in half by 1998. At least five follow-up agreements since then helped to deepen the cuts, advanced timetables for compliance, and addressed additional ozone-depleting substances such as halons, methyl chloroform, carbon tetrachloride, and hydrochlorofluorocarbons (HCFCs). Most countries around the world have phased out production of the substances covered by the agreements and industry has been able to shift to safer alternative chemicals. As a result, there’s evidence that the Antarctic ozone hole has stopped growing worse, although recovery is not expected anytime soon. Phasing out CFCs and HCFCs is also beneficial in protecting the earth's climate, as these substances are also very damaging greenhouse gases.
As part of the United States' commitment to implementing the Montreal Protocol, the U.S. Congress amended the Clean Air Act (section 6.7), adding provisions for protection of the ozone layer. Most importantly, the amended Act required a gradual end to the production of chemicals that deplete the ozone layer. The Clean Air Act amendments passed by Congress require the Environmental Protection Agency (EPA) to develop and implement regulations for the responsible management of ozone-depleting substances in the United States. Under the Clean Air Act, EPA has created several regulatory programs to address numerous issues, including:
- ending the production of ozone-depleting substances,
- ensuring that refrigerants and halon fire extinguishing agents are recycled properly,
- identifying safe and effective alternatives to ozone-depleting substances,
- banning the release of ozone-depleting refrigerants during the service, maintenance, and disposal of air conditioners and other refrigeration equipment,
- requiring that manufacturers label products either containing or made with the most harmful ozone-depleting substances.
ADAPT \(\PageIndex{1}\)
ADAPT \(\PageIndex{2}\)
ADAPT \(\PageIndex{3}\)
ADAPT \(\PageIndex{4}\)
Summary
- Any disruption of the balance that results in a higher rate of ozone destruction than ozone creation would result in the depletion of ozone.
- Some compounds that release chlorine or bromine when they are exposed to intense UV light in the stratosphere contribute to ozone depletion and are called ozone-depleting substances (ODS)
- The Montreal Protocol is an international agreement that committed all parties (signatory nations) to a schedule for phasing out the production and use of CFCs and other substances known to be harmful to the ozone layer.
Contributors and Attributions
- Libretext: Supplemental Module, Physical and Theoretical Chemistry
- Caralyn Zehnder et al.
- Erin Avram (Cleveland State University)

