5.1: Enzymes
- Page ID
- 234006
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Explain the functions of enzymes.
- Explain how enzymes are classified and named.
A catalyst is any substance that increases the rate or speed of a chemical reaction without being changed or consumed in the reaction. Enzymes are biological catalysts, and nearly all of them are proteins. The reaction rates attained by enzymes are truly amazing. In their presence, reactions occur at rates that are a million (106) or more times faster than would be attainable in their absence. What is even more amazing is that enzymes perform this function at body temperature (~37°C) and physiological pH (pH ~7), rather than at the conditions that are typically necessary to increase reaction rates (high temperature or pressure, the use of strong oxidizing or reducing agents or strong acids or bases, or a combination of any of these). In addition, enzymes are highly specific in their action; that is, each enzyme catalyzes only one type of reaction in only one compound or a group of structurally related compounds. The compound or compounds on which an enzyme acts are known as its substrates.
Hundreds of enzymes have been purified and studied in an effort to understand how they work so effectively and with such specificity. The resulting knowledge has been used to design drugs that inhibit or activate particular enzymes. An example is the intensive research to improve the treatment of or find a cure for acquired immunodeficiency syndrome (AIDS). AIDS is caused by the human immunodeficiency virus (HIV). Researchers are studying the enzymes produced by this virus and are developing drugs intended to block the action of those enzymes without interfering with enzymes produced by the human body. Several of these drugs have now been approved for use by AIDS patients.
| Class | Type of Reaction Catalyzed | Examples |
|---|---|---|
| oxidoreductases | oxidation-reduction reactions | Dehydrogenases catalyze oxidation-reduction reactions involving hydrogen and reductases catalyze reactions in which a substrate is reduced. |
| transferases | transfer reactions of groups, such as methyl, amino, and acetyl | Transaminases catalyze the transfer of amino group, and kinases catalyze the transfer of a phosphate group. |
| hydrolases | hydrolysis reactions | Lipases catalyze the hydrolysis of lipids, and proteases catalyze the hydrolysis of proteins |
| lyases | reactions in which groups are removed without hydrolysis or addition of groups to a double bond | Decarboxylases catalyze the removal of carboxyl groups. |
| isomerases | reactions in which a compound is converted to its isomer | Isomerases may catalyze the conversion of an aldose to a ketose, and mutases catalyze reactions in which a functional group is transferred from one atom in a substrate to another. |
| ligases | reactions in which new bonds are formed between carbon and another atom; energy is required | Synthetases catalyze reactions in which two smaller molecules are linked to form a larger one. |
The first enzymes to be discovered were named according to their source or method of discovery. The enzyme pepsin, which aids in the hydrolysis of proteins, is found in the digestive juices of the stomach (Greek pepsis, meaning “digestion”). Papain, another enzyme that hydrolyzes protein (in fact, it is used in meat tenderizers), is isolated from papayas. As more enzymes were discovered, chemists recognized the need for a more systematic and chemically informative identification scheme. In the current numbering and naming scheme, under the oversight of the Nomenclature Commission of the International Union of Biochemistry, enzymes are arranged into six groups according to the general type of reaction they catalyze (Table \(\PageIndex{1}\)), with subgroups and secondary subgroups that specify the reaction more precisely.
Figure \(\PageIndex{1}\): Structure of the alcohol dehydrogenase protein (E.C.1.1.1.1) (EE ISOZYME) complexed wtih nicotinamide adenini dinulceotide (NAD) and zinc (PDB: 1CDO).
Each enzyme is assigned a four-digit number, preceded by the prefix EC—for enzyme classification—that indicates its group, subgroup, and so forth. This is demonstrated in Table \(\PageIndex{2}\) for alcohol dehydrogenase. Each enzyme is also given a name consisting of the root of the name of its substrate or substrates and the -ase suffix. Thus urease is the enzyme that catalyzes the hydrolysis of urea.
| Alcohol Dehydrogenase: EC 1.1.1.1 | |
|---|---|
| The first digit indicates that this enzyme is an oxidoreductase; that is, an enzyme that catalyzes an oxidation-reduction reaction. | |
| The second digit indicates that this oxidoreductase catalyzes a reaction involving a primary or secondary alcohol. | |
| The third digit indicates that either the coenzyme NAD+ or NADP+ is required for this reaction. | |
| The fourth digit indicates that this was the first enzyme isolated, characterized, and named using this system of nomenclature. | |
| The systematic name for this enzyme is alcohol:NAD+ oxidoreductase, while the recommended or common name is alcohol dehydrogenase. | |
|
Reaction catalyzed: |
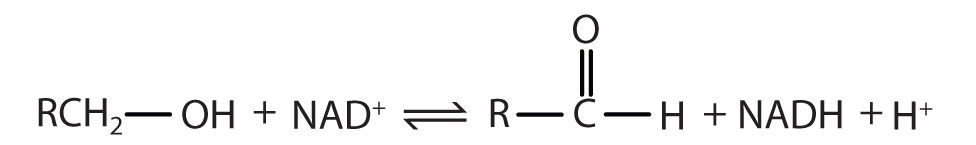 |
Common Nomenclature
The systematic names are informative but not very practical. Therefore, most chemists still use the common nomenclature system. In this system, enzyme names consists of either one of these two options:
-
name of the substrate + “ase”. Example: alcohol dehydrogenase.
-
substrate name + functional group action on + type of reaction + “ase”. Example: Urea amidohydrolase.
Quantifying Enzyme concentration
The quantity or concentration of an enzyme in a solution can be expressed in molarity amounts, as with any other chemical. However, this is not the most practical option. Being proteins, enzymes have very large molar masses so their concentration in terms of molarity is extremely low. Instead, it is more useful to measure enzyme concentration in terms of the enzymatic activity exhibited by the enzyme in a certain volume of solution. This second option is preferred as the amount of enzyme is usually very low in terms of moles or grams, but its activity is very high and easier to determine.
Enzyme international unit (symbol U, sometimes also IU) is a unit of enzyme's catalytic activity. This unit is defined as the amount of the enzyme that catalyzes the conversion of one micromole of substrate per minute under the specified conditions. The specified conditions will usually be the enzyme optimum conditions, which including temperature, pH, and substrate concentration, that yield the maximal substrate conversion rate for that particular enzyme.
1 U = 1 μmol substrate/min
Characteristics of enzymes
Chemically, enzymes are generally globular proteins. Some RNA molecules called ribozymes can also be enzymes. These are usually found in the nuclear region of cells and catalyze the splitting of RNA molecules. Enzymes are catalysts that breakdown or synthesize more complex chemical compounds. Enzymes are characterized by the following factors:
1-Efficiency. Enzymes allow chemical reactions to occur fast enough to support life. They speed up the rate of chemical reactions because they lower the energy of activation, the energy that must be supplied in order for molecules to react with one another. An enzyme generally can typically catalyze between 1 and 10,000 molecules of substrate per second. In contrast to traditional chemical catalysts, enzymes are able to perform their action at body temperature and physiological pH.
2-Specificity. Enzymes are highly specific for their substrate. Generally there is one specific enzyme for each specific chemical reaction.
3-Regulation. The activity of enzymes is highly regulated in the cell to adapt to different metabolic and environmental conditions. The activity of an enzyme can be altered by either changing the total amount of enzyme (enzyme concentration in the cell) or by changing the rate at which an enzyme can catalyze a chemical reaction (enzymatic activity).
Summary
An enzyme is a biological catalyst, a substance that increases the rate of a chemical reaction without being changed or consumed in the reaction. A systematic process is used to name and classify enzymes.
Concept Review Exercise
In the small intestine, sucrose is hydrolyzed to form glucose and fructose in a reaction catalyzed by sucrase.
- Identify the substrate in this reaction.
- Name the enzyme.
Answers
- sucrose
- sucrase
Exercises
-
Identify the substrate catalyzed by each enzyme.
- lactase
- cellulase
- peptidase
-
Identify the substrate catalyzed by each enzyme.
- lipase
- amylase
- maltase
-
Identify each type of enzyme.
- decarboxylase
- protease
- transaminase
-
Identify each type of enzyme.
- dehydrogenase
- isomerase
- lipase
Answers
-
- lactose
- cellulose
- peptides
-
- lyase
- hydrolase
- transferase
Citations
- Wikipedia contributors. (2020, November 28). Enzyme unit. In Wikipedia, The Free Encyclopedia. Retrieved 17:34, May 17, 2021, from https://en.wikipedia.org/w/index.php?title=Enzyme_unit&oldid=991101591
- Enzymes. (2020, August 17). Retrieved May 17, 2021, from https://chem.libretexts.org/@go/page/16020. " Enzymes" by LibreTexts is licensed under CC BY-NC-SA .
- Enzymes. (2021, January 3). Retrieved June 22, 2021, from https://bio.libretexts.org/@go/page/3427. " Enzymes" by Gary Kaiser, LibreTexts is licensed under CC BY .

