3.6: Alkene Asymmetry and Markovnikov's Rule
- Page ID
- 221777
Learning Objective
- predict the products/specify the reagents for EAR of hydrohalic acids (HX) with asymmetrical alkenes using Markovnikov's Rule for Regioselectivity
- apply the principles of regioselectivity and stereoselectivity to the addition reactions of alkenes
Addition to unsymmetrical alkenes
In terms of reaction conditions and the factors affecting the rates of the reaction, there is no difference whatsoever between these alkenes and the symmetrical ones described above. The problem comes with the orientation of the addition of asymmetric reactants, such as H2O and HX, across the double bond.
Markovnikov's Rule
If an asymmetric reactant, such as HCl, adds to an unsymmetrical alkene like propene, there are two possible ways it could add. However, in practice, there is only one major product according to Markovnikov's Rule.
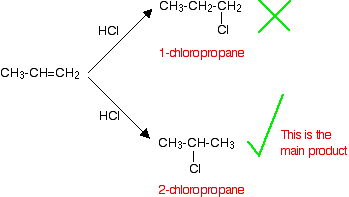
Markovnikov's Rule: When an symmetric reactant is added to an unsymmetrical alkene, the hydrogen becomes attached to the carbon with the most hydrogens attached to it already.
Applying Markovnikov's Rule to the reaction above, the hydrogen bonds with the CH2 group, because the CH2 group has more hydrogens than the CH group. Notice that only the hydrogens directly attached to the carbon atoms at either end of the double bond count.
This type of reaction is called regioselective, because the breaking of the double bond favors one direction over another, resulting in a specific isomer as the major product (2-chloropropane is obtained with a larger yield compared to 1-chloropropane, instead of the expected 50:50 mixture between these two possible products)
Markovnikov's Rule - a closer look
To understand the bases of Markovnikov's Rule, we need to consider the reaction mechanism for the addition of HX or water to the double bond. A reaction mechanism is the step-by-step sequence of elementary reactions by which overall chemical change occurs.
The mechanism for this reaction consists of three steps
Step 1. The acid HX dissociates into H+ and X-
H—Cl → H+ + Cl-
Step 2. The H⁺ (an electrophile) to the double bond (a nucleophile). This generates a carbon atom with a positive charge known as a carbonation. Two possible carbonation intermediates can be formed from propene:
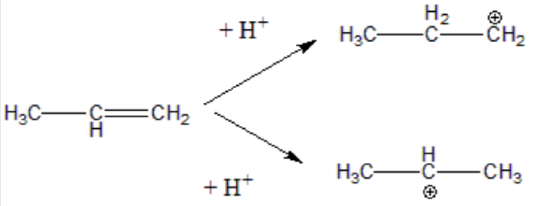
Not all carbocations have the same stability. The stability order for carbocation decreases as shown:
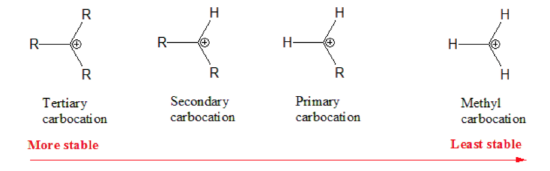
Therefore, during this second step, the formation of a secondary carbonation is favored (more stable than the primary carbonation):
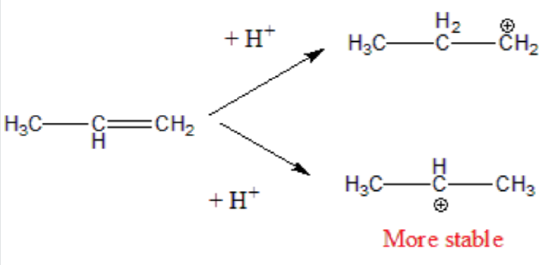
Step 3. The carbocation is very reactive intermediate, and it combines with the halide X- anion forming the final addition product. The major product derives from the most stable carbonation.
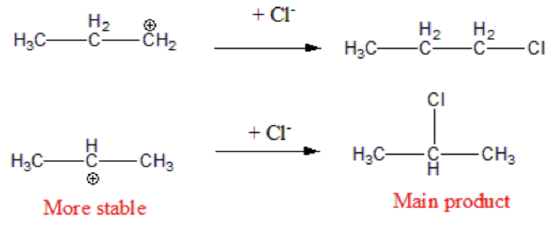
Preferred formation of the most stable carbocation intermediate is the basis for Markovnikov's Rule and the regioselectivity of this reaction. Remember that something very similar occurs with the addition of H2O in the presence of an acid catalyst. In this case, the acid catalyst is responsible for initiation step 1 in the mechanism.
Exercises
1. Predict the product(s) for the following reactions:
2. In each case, suggest an alkene that would give the product shown.
- Answers
-
1.
2.
Citations, Contributors and Attributions
Alkene Asymmetry and Markovnikov’s Rule. (2020, May 30). Retrieved May 20, 2021, from https://chem.libretexts.org/@go/page/110139
Wikipedia contributors. (2021, January 2). Regioselectivity. In Wikipedia, The Free Encyclopedia. Retrieved 16:14, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Regioselectivity&oldid=997903090
Wikipedia contributors. (2021, March 14). Reaction mechanism. In Wikipedia, The Free Encyclopedia. Retrieved 16:14, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Reaction_mechanism&oldid=1012125264
Wikipedia contributors. (2021, January 7). Markovnikov's rule. In Wikipedia, The Free Encyclopedia. Retrieved 16:15, May 20, 2021, from https://en.wikipedia.org/w/index.php?title=Markovnikov%27s_rule&oldid=998882337
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)
- Gamini Gunawardena from the OChemPal site (Utah Valley University)

