1.7: Back to Hemp Seed Oil
- Page ID
- 424905
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)To restate an important point from the previous sections, the effects of making seemingly small structural changes to compounds can have drastic effects on their chemical and physical properties. We chose these particular groups, the carbon-carbon double bonds of alkenes, the hydroxyl groups of alcohols, and the carboxylic acid moieties, because of their relevance to understanding the structure of linoleic acid, but others could demonstrate the concepts just as effectively.
Returning to hemp seed oil and its major component, linoleic acid, we are now in a position to more meaningfully consider what this particular chemical is and what it may do. We can begin by dissecting its systematic name, (9Z,12Z)-octadecadienoic acid. Let’s unpack the information embedded in this less than mellifluous name. As we already know, the main chain is eighteen carbon atoms long, as indicated by the “octadeca-” prefix. In addition, the presence of the two double bonds is communicated by the “-dien-”, and these begin at carbons nine and twelve. The presence of the hydroxyl group on the first carbon does not mean this compound is an alcohol because, as we indicated, the OH is adjacent to a carbonyl group, which makes the compound a carboxylic acid, hence the “-oic acid” appendage. The only remaining information concerns those curious Z’s that follow the 9 and 12 “addresses” of the double bonds. This is a variation on the cis terminology we already encountered; we will describe it more completely later but, for now, the “9Z,12Z” appellation conveys the specific geometry about the double bonds at carbons 9 and 12: they both have the cis configuration (trans would designated with an “E”). You can now see that the name (9Z,12Z)-octadecadienoic acid provides you with all of the information you require to know its molecular structure. Common names, such as linoleic acid, are just that, common, meaning they are frequently used, especially by those who use a particular chemical frequently, but they lack the structural information that the systematic names provide.

Figure 1-35. Stepwise hydrogenation of linoleic acid (a polyunsatutrated fatty acid), first to oleic acid (a monounsaturated fatty acid), then finally to stearic acid (a saturated fatty acid). Notice that the general shape of the molecules changes as a result of the addition of hydrogen: linoleic acid is hook shaped, while stearic acid shaped more like a straight rod; these differences in shape turn out to be important for understanding the structure and function of cell membranes.
Can we understand something about the properties of linoleic acid based on its structure? By all means, yes. For instance, we described it earlier as an oily liquid. The oiliness, which essentially is a way of simultaneously conveying its hydrophobicity and its viscosity, the latter term referring to the fact that it is “thick” compared to other liquids and resists flow. Let’s apply what we have already presented concerning the effects of functional groups to this one compound to illustrate the usefulness of the approach in developing an intuition, for lack of a better word, of how chemicals “should” behave. First, the structure has two distinct portions: a long tail that is essentially an unsaturated hydrocarbon, we expect this portion of the molecule to be hydrophobic, and a “head” consisting of what we expect to be a hydrophilic carboxylic acid moiety. Such structures are called amphiphilic because they simultaneously love polar and nonpolar environments, albeit with different parts of their structures. Soaps and detergents have similar structures (as do the major components of cell membranes) and we would therefore expect linoleic acid to behave similarly to such materials, and this is borne out by experiment. Because the hydrophobic tail is so much larger than the hydrophilic head, we might also predict that linoleic acid has limited solubility in water. This is true and is the basis for its “oily” character.
We might also surmise, given the two double bonds, that linoleic acid would have a greater reactivity toward oxidizing agents. There is evidence that, taken as a dietary supplement, linoleic acid does, in fact, have antioxidant properties, sacrificially reacting with oxygen species in order to protect other alkene functionalities in various membrane structures. It can also be converted via hydrogenation to oleic acid (so named because it is the major component of olive oil) by the addition of one H2 molecule (Figure 1-35). Linoleic acid, because it has more than one carbon-carbon double bond, is referred to as a polyunsaturated fatty acid, whereas oleic acid is a monounsaturated fatty acid. Addition of a second molecule of hydrogen saturates the carbon chain with hydrogen atoms and yields stearic acid, a saturated fatty acid.
It is worth turning our attention back to the concept of “natural” and “artificial” chemicals at this point. The stearic acid we just mentioned is often prepared commercially by reacting fats obtained from plant oils and animal fats with metal catalysts and hydrogen gas (H2) which, in turn, is usually derived from crude oil. But stearic acid can also be found naturally in many animals and plants as well as inside you. Is there any difference between “natural” stearic acid and artificially obtained stearic acid? The list of ingredients on Burt’s Bees Chemical-Free Sunscreen includes stearic acid. Should it indicate if that was obtained from a natural source or prepared by artificial hydrogenation of linoleic or oleic acid? The molecules themselves are identical in every way so, as far as your body is concerned, the source of the fatty acid is of no consequence whatsoever. That generated naturally is just as rejuvenating when applied to your skin, and unhealthy when ingested in excess, as that produced in a fat processing facility.
The question of natural versus artificial can be legitimate, however. Is it better to take a tablet of synthetically produced vitamin C (ascorbic acid), or eat an orange? The orange comes with a host of other materials that aid in digestion and can be healthy in other ways. Plus, they’re tasty. The tablet has binders and other materials along with the vitamin C, and these other additives may or may not have any health benefits. So, no, taking a vitamin C tablet is not the same as eating an orange. But the difference is definitely not related to the natural or synthetic origins of the vitamin C per se. A molecule of ascorbic acid is the same no matter who or what synthesized it or by what means. It is a chemical, like linoleic acid. And chemicals, once understood, provide no cause for the reflexive recoil exhibited by many people. Some are dangerous and require respect, but all are fascinating, having their own personalities and stories to tell. Stick with us and we’ll share some in chapters that follow.

Figure 1-36. Natural or not? And what difference does it make? The molecules of vitamin C (structure shown) found in dietary supplements are identical in every way to those found in oranges, strawberries, and other fruits. There is no way of telling where or how a specific molecule was made. That said, a diet rich in fruits and vegetables has all sorts of benefits, but those have nothing to do with how natural or artifical the vitamins and minerals in them are. It has everything to do with what else comes along for the ride.
(Photo credits: "Medicine box with Vitamine C pills above white background" by wuestenigel is licensed under CC BY 2.0; "Orange fruit" by piropiro3 is licensed under CC BY 2.0.)
Additional Problems For Chapter 1
1-21) What is the molecular formula for each of the compounds represented by the structural diagrams below.
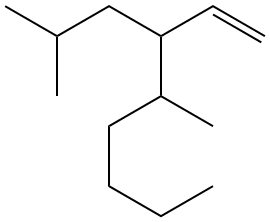
1.22) Determine the molecular formula for each of the line drawings below.
1.23) Draw the structures of the following compounds.
a) 2,3-dimethylhexane
b) 3-ethylhexane
c) 2,2,3,3-tetramethylbutane
1.24) Which, if any, of the compounds above are isomers of each other?
1.25) Name the following alkanes
1.26) The name 2-ethylpentane is invalid but you can nevertheless draw a structure that corresponds to that name. Draw it and name it correctly.
1.27) The four isomeric forms of -C4H9 alkyl groups were presented in Figure 1-17. Draw four isomeric forms of C14H30 by placing each of these groups on a ten carbon chain and name each compound.
1.28) List all of the possible locants for isomers of C14H30 having the following names (where # represents a valid locant):
a) #-butyldecane
b) #-tert-butyldecane
c) #-(2-methylpropyl)decane
d) #-(butan-2-yl)decane
1.29) Draw the structure of the isopentyl alkyl group. What is the systematic name of this alkyl group
1.30) Figure 1-17 shows all four isomeric forms of alkyl groups with the formula -C4H9. Draw and name all of the isomeric forms for alkyl groups with the formula -C5H11. Which one would have the informal name “isopentyl”?.
1.31) Speculate why carboxylic acids have higher boiling points than alcohols having the same number of carbon atoms.
1.32) What is the product of the addition of hydrogen (H2) to hept-2-ene? What are the possible products of the addition of water to hept-2-ene?
1.33) Why is the name but-3-ene incorrect? What would be the correct name for this structure? Is cis/trans isomerism possible for this structure?
1.34) Give IUPAC names for the following alkenes.
|
a)
|
b)
|