1.6C: Alkenes
- Page ID
- 424839
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkenes. The final functional group we present in this brief introduction to the topic is the carbon-carbon double bond. Compounds exhibiting such a structural feature are called alkenes and are designated with an –ene suffix in their names. Alkenes and alkanes are both examples of hydrocarbons, compounds that are composed solely of carbon and hydrogen. The hydrophobicity and relatively low density of alkanes are typical of all hydrocarbons, including alkenes. The chemical properties of various types of hydrocarbons can differ more substantially, however, and alkenes are often more reactive than alkanes, as discussed below.
Alkanes are often referred to as saturated hydrocarbons, meaning that the carbon atoms are bonded to as many hydrogen atoms as possible. Just as a saturated sponge holds as much water as possible, a carbon framework that is saturated “holds” as many hydrogen atoms as it can given the requirement that carbon makes four bonds to neighboring atoms. If some hydrogen atoms were to be removed (somehow), carbon would satisfy its need to form four bonds by making multiple bonds with other carbon atoms. Such a compound would be an unsaturated hydrocarbon, meaning that additional hydrogen atoms could be accommodated by the existing carbon framework. All alkenes are unsaturated hydrocarbons.
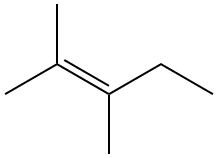
There are a number of subtleties that arise when considering the structures of alkenes that don't exist for alkanes. Let’s perform a simple thought experiment that would convert butane, which is saturated, to butene, which is unsaturated, by removing hydrogen atoms on adjacent carbons. There are two ways we can do this: the hydrogen atoms can be removed from the first and second carbon atoms, or from the second and third. These two possibilities are illustrated in Figure 1-25; the hydrogen atoms we will pluck off are shown in red, and the double bond will form between the two carbons whose hydrogens are removed. The position of the resulting double bond, therefore, will depend on which hydrogens are removed. These products, but-1-ene and but-2-ene, are isomers: they have the same molecular formulas, C4H8 but different structures. A discussion of how alkenes are named is presented in just a bit.

Figure 1-25: The hypothetical conversion of butane to two isomers of butene. The left pathway yields but-1-ene, while the right yields but-2-ene, the cis isomer of which is shown; the trans isomer, not shown, could also be generated (see text). Line diagrams are provided underneath the structural formulas to offer a different depiction of the structures.
An important point about double bonds is that they are structurally much more rigid than single bonds. Atoms connected by single bonds can usually rotate easily around those bonds. The terminal methyl groups on butane, for example, rapidly spin like pinwheels at room temperature. Such rotations are not limited to terminal groups and give rise to a flexibility all along the carbon backbones of alkanes; liquid alkanes, therefore, are constantly jiggling and changing shape, much like a string of beads can change shape without coming apart.[17] In contrast, the atoms associated with the double bonds of alkenes lie in the same geometric plane and show no such rotational motion. In other words, the six atoms around a given double bond, i.e., the two carbon atoms directly bonded plus the four other atoms directly connected to those carbons, lie in a flat plane and strongly resist any motion that disrupts takes them out of that plane. This can clearly be seen in the simplest alkene, C2H4, which goes by the IUPAC name ethene, a model of which is seen from two perspectives in Figure 1-26. The top view is looking down on the plane of the molecule; all the atoms are sitting in the plane of the page (or screen). The second view is along the plane; you can see both carbon atoms and two hydrogen atoms clearly and they all lie in the same plane. The remaining two hydrog en atoms are mostly hidden behind the other atoms, but you if you observe closely, you get a "peek-a-boo" view of them. The planarity of the carbon-carbon functional group gives rise to important structural consequences as described below.
en atoms are mostly hidden behind the other atoms, but you if you observe closely, you get a "peek-a-boo" view of them. The planarity of the carbon-carbon functional group gives rise to important structural consequences as described below.
Figure 1-26: Two views of the simplest alkene, C2H4. The top view (a) shows all six atoms; they are all in the plane of the page. The bottom view (b) is perpendicular to the top and views the molecule along the plane of the molecule; the coplanar.ity of all six atoms is more clearly evident in this view.
This lack of rotation around the double bond can result in a type of isomerism we have not seen yet. The two isomers of but-2-ene are illustrated in Figure 1-27. The sort of isomerism in this case is distinctly different from the sort we encountered with, for example, 2-methylpentane and 3-methylpentane. In that case, the ordering of the atoms along the chain was different; the structures differed by virtue of the location of the methyl substituents along the pentane chain. Accordingly, this sort of isomerism is called structural isomerism (also called constitutional isomerism), so termed because the structures, that is, the way the component atoms are connected to each other, are not the same. Similarly, the isomerism exhibited in Figure 1-25, between but-1-ene and but-2-ene, is also structural because the position of the double bond differs between the two structures. But in the case of the two isomers of but-2-ene shown in Figure 1-27, the position of the functional group is identical: the double bond begins with the second carbon along the chain.
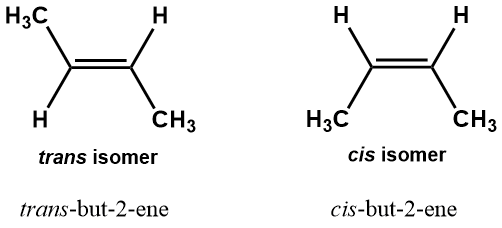
Figure 1-27. Cis and trans isomers of but-2-ene. These are geometric isomers, meaning that the order of atoms and bonds along the chain is identical but their arrangement in space differs.
The difference between the two structures shown above lies in how the groups extending from the double bond are situated with respect to each other. You can easily notice the difference if you draw a line between the two methyl groups attached to the double bond (Figure 1-28): in the structure on the right, that line cuts across the double bond, whereas in the structure on the left, the two methyl groups are located on the same side (they are both positioned below the double bond); the connecting line does not cross the double bond, but stays beneath it. When the two carbons of the main chain lie on opposite sides of the double bond they are said to be trans to each other, trans- being the commonly used prefix to indicate “across”, and the compound is referred to as the trans isomer. Hence the complete name is trans-but-2-ene. The other configuration has the two methyl groups on the same side and is referred to as the cis isomer, making the full name cis-but-2-ene; this is the isomer shown in Figure 1-25. These molecules are examples of geometric isomers, defined as those having the same atomic “connectivity”, meaning the order in which atoms are connected in the molecule, but different spatial relationships between the component atoms. In this case, both molecules have four carbon chains with a double bond between the second and third carbon atoms, but the spatial relationship between the atoms connected to the doubly-bonded carbons are not the same. This can lead to significant differences in molecular shapes that can have surprisingly pronounced physiological consequences. The current controversy over so-called trans fats is directly related to this idea. We will see other types of geometric isomerism later in this text.
 |
| Figure 1-28. Drawing a line between the two methyl groups of but-2-ene illustrate the difference between cis and trans structures. In the cis isomer, the two methyl groups are on the same side of the double bond, which you can see because the line drawn between them does not cut across the double bond itself. In contrast, the two methyl groups of the trans isomer are on opposite sides of the double bond; a line connecting them must cross the double bond. |
Finally, before leaving the topic of alkene isomers, it is worth pointing out that the mere presence of a double bond does not necessitate the existence of cis and trans isomers. But-1-ene, for example, exists in only one form – it has no cis or trans isomers. Why? For cis/trans isomerism to exist, it is necessary that neither carbon atom of the double bond be connected to two identical groups. That is, if either carbon is connected two identical substituents, no geometric isomerism is possible. To see the reason consider the following test: if you swap the positions of the two substituents on one – and only one – carbon of the double bond and thereby generate a structure in which the substituents have a different spatial relationship with the substituents of the other carbon of the double bond, then you have generated a new structure, one that is distinct from the original form. An isomer, in other words.. In but-1-ene, for example, the carbon in the one position is bound to two hydrogen atoms; swapping their positions does not yield a new species, but one that is identical to the original - all you have done is switch the position of two identical atoms, making it indistinguishable from the original structure. In but-2-ene, on the other hand, swapping the positions of the H and CH3 bound to the carbon in the second position will transform the cis to trans (or trans to cis) isomers. To summarize: cis/trans isomerism can only exist when each carbon atom on a double bond is connected to two different substituents .
If the above is unclear to you, some students find a more logical (code-like ?) approach is helpful here. Refer to Figure 1-29. In this figure, the pairs of substituents on each carbon of the double bond are labeled R and R′ on the left carbon, and R′′ and R′′′ on the right. Geometric isomers will only exist if both of the following conditions are true: R ≠ R′ and R′′ ≠ R′′′. In the case of but-2-ene, R = H, R′ = -CH3, R′′ = H and R′′′ = -CH3, satisfying the condition for geometric isomerism; it doesn't matter that R = R′′ or that R′ = R′′′ because those relationships are not relevant for the existence (or lack thereof) of cis/trans isomerism. In the case of but-1-ene, R = R′ = R′′ = H and R′′′ = -CH2CH3. The fact that R = R′ means that no geometric isomers of but-1-ene exist.
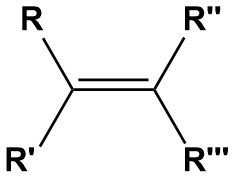
Figure 1-29. Geometric isomerism can exist only when R ≠ R’ and R’’ ≠ R’’’. Here, the R groups can be any alkyl group or a hydrogen atom. For example, cis/trans isomerism can exist if R = H, R’ = methyl and R’’ = methyl and R’’’= ethyl, but not when R = H, R’ = methyl and R’’ = R’’’= methyl. In the latter case, because R’’ = R’’’, there will only be one structure possible with the specified connectivity.
Nomenclature: Alkenes
We’ll illustrate the naming of alkenes with the two examples below. The basic rules are similar to what we introduced for alkanes and alcohols, with a few modifications. As with alkanes, you first need to identify the longest chain of carbon atoms, regardless of whether or not that chain contains the double bond. The manner by which the presence of the double bond is reflected in the name of the compound depends on whether or not the longest chain contains the double bond. For example, the two structures below are the same in that the longest chain in each has seven carbon atoms; the double bond is in the longest chain in the structure on the left, but it is not in the compound at right.

Starting with the structure on the left, when the longest chain contains the double bond the molecule is named as follows:
-
The name of the alkene will be based on the length of the longest chain, with -ene as a suffix, in place of the -ane for the alkane of the same length. In addition, the position of the double bond is indicated by a locant that immediately precedes the -ene suffix; this number indicates the carbon at which the double bond starts. Number the chain in such a way that the locant is the smallest number possible. The molecule on the left will be named as a substituted hept-2-ene.
-
Determine the type and position of each substituent on the longest chain. At this point we know the name will include "4-ethylhept-2-ene."
-
Determine if cis/trans isomerism is possible and, if so, determine which form the molecule is in. Here, the two alkyl groups are on the same side of the molecule, making it the cis isomer. The full name is therefore cis-4-ethylhept-2-ene.
What about when the longest chain does not contain the double bond? Fair warning: naming compounds where the double bond is not part of the longest chain can get a bit complicated. It takes some practice getting used to the cumbersome names they can have, but it isn't impossible. Such compounds are named as alkane derivatives where a substituent name indicates the presence of a double bond. The compound above (at right), is therefore named as heptane derivative, but rather than having an ethyl substituent, which would be -C2H5, the substituent is named ethenyl, where the -enyl indicates a double bond the the two carbon fragment; specifically, it is the -en- piece of the substituent's suffix signifies the presence of the double bond. The name is therefore 4-ethenylheptane.
As the above suggests, a new set of substituent names is necessary if a double bond is present. Instead of providing an exhaustive list (which is not even possible), a few simple examples will suffice. We will limit our discussion to substituents of three or fewer carbon atoms as the substituent names quickly become quite cumbersome, but you can glean the pattern that is developed with these examples.
 The first thing to consider when naming compounds of this sort: is the substituent connected to the main chain via a single bond or a double bond? Examples of the latter type are illustrated in Figure 1-30; this shows one-, two-, and three-carbon substituents bound to a long chain via a double bond. The substituents are named methylidene, ethylidene, and propylidene, respectively. The -ylidene suffix is reserved for substituents bound to longer chains via double bonds.
The first thing to consider when naming compounds of this sort: is the substituent connected to the main chain via a single bond or a double bond? Examples of the latter type are illustrated in Figure 1-30; this shows one-, two-, and three-carbon substituents bound to a long chain via a double bond. The substituents are named methylidene, ethylidene, and propylidene, respectively. The -ylidene suffix is reserved for substituents bound to longer chains via double bonds.
Figure 1-30. Examples of compounds having substituents bond to a long chain via a double bond.
The three examples in Figure 1-30 feature simple, unbranched substituents, but other substituents can also be connected to a long chain via a double bond and these are also named using the -ylidene suffix. Figure 1-31 shows a 3-carbon substituent that is connected at its central carbon to a nine-carbon chain via a double bond. The IUPAC name for the structure shown is 4-(propan-2-ylidene)nonane. The "propan" part of the name indicates that it is the substituent is 3 carbon atoms and has no double bonds along its length; the "2" preceding the -ylidene suffix indicates that the substituent is connected to the main chain at its second carbon and, as previously stated, the -ylidene suffix indicates that it is connected to the rest of the molecule via a double bond. Note that the name of the substituent is in parentheses that follow the locant "4", meaning that the substituent is connected on the fourth carbon of the nine-carbon chain; the parentheses serve to clarify that the name within them is a single substituent connected at the immediately preceding locant.
Figure 1-31. An example of a branched substituent  connected to a long chain via a double bond.
connected to a long chain via a double bond.
When substituents that contain a double bond are connected to the main structure via a single bond, the name of the compound must specify three structural features: 1) where the substituent is attached along the main chain; 2) the position of the double bond along the substituent chain, and; 3) which carbon of the substituent is the point of attachment to the main chain. To illustrate, we'll adapt the structure shown in Figure 1-31 by shifting the position of the double bond (Figure 1-32). The new compound is named 4-(prop-1-en-2-yl)nonane. Notice that the three locants correspond to the three pieces of information we need: the "4" indicates where along the main chain the substituent is attached; the "1" preceding the -en indicates that the double bond starts at carbon number one of the substituent, and; the "2" indicates that it is the second carbon of the substituent that is the point of attachment to the main chain. In terms of numbering the substituent, the locant indicating the point of attachment takes priority over the position of the double bond, meaning that the substituent chain is numbered to make the former number as small as possible.

Figure 1-32. The three isomeric forms of the propenyl functional group, C3H5, that is connected to a larger chain via a carbon-carbon single bond. The numbering schemes and for the main chain and substituent chain are shown in red and blue, respectively. Note that the numbering of the substituent chain prioritizes the oint of attachment not the position of the double bond.
Table 1-7 summarizes the naming for alkenyl substituents having up to 3 carbons. Based on the principles described above, however, you should be able to name larger groups, although they are not frequently encountered.
| Table 1-7: Names and Structures of Alkenyl Substituents Up To 3 Carbon Atoms In Length | ||
| Number of Carbon Atoms of Substituent | Structure | Name |
| 1 | =CH2 | methylidene |
| 2 | =CH-CH3 | ethylidene |
| 2 | -CH=CH2 | ethenyl |
| 3 | =CH-CH2-CH3 | propylidene |
| 3 | -CH=CH-CH3 | prop-1-en-1-yl |
| 3 | -CH2-CH=CH2 | prop-2-en-1-yl |
| 3 | =C(CH3)2 | propan-2-ylidene |
| 3 | -C(CH3)=CH2 | prop-2-en-2-yl |
Problem 1-18. Putting it all together: what is the systematic name for the structure shown at right? 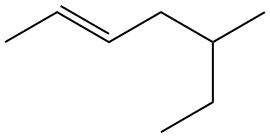
Solution
-
Identify the longest chain: seven carbons.
-
The double bond is contained within the main chain, so the name will end in heptene.
-
Establish numbering system: we begin the numbering with the end of the chain that is closest to the double bond; the double bond starts at the second carbon. So the name will have hept-2-ene after listing the substituents.
-
There is only one substituent off the chain, a methyl group on the fifth carbon, making our name (so far) 5-methyl-2-heptene.
Indicate whether or not this is a geometric isomer of another compound with the same connectivity. Yes, it is. This is the trans isomer (carbons 1 and 4 are across the double bond from each other. So the complete name is trans-5-methylhept-2-ene.
Worked Problem 1-19. Putting it all together, redux: what is the systematic name for the following compound? 
Solution
-
Identify the longest chain: seven carbons.
-
The double bond is not contained within the main chain, so the name will end in heptane.
-
Establish numbering system: we begin the numbering with the end of the chain that is closest to the only substituent. The name will be 3-xheptane, where x is a placeholder for the substituent name.
-
The substituent has just one-carbon and is connected to the main chain via a double bond; referring to the table above, its name is methylidene. The name (so far) is 3-methylideneheptane.
Indicate whether or not this is a geometric isomer of another compound with the same connectivity. No, it isn’t because there are two hydrogens on the same side of the double bond. The complete name is therefore 3-methylideneheptane.
Problem 1-20. Provide IUPAC names for each of the following compounds, all of which have the molecular formula C7H14.
|
a)
|
c)
|
|
b)
|
d)
|
We have now spent considerable time focusing on the structures of alkenes but, if the only difference between alkanes and alkenes were the structural features described above, they would not be particularly interesting from a chemical perspective. As we mentioned briefly earlier, however, the presence of the double bonds makes possible a variety of chemical reactions that are not available to alkanes. This is because the electrons – recall that these are the negatively charged particles that chemical bonds consist of – that comprise double bonds are less tightly held by molecules than those in single bonds. [18] One consequence is that double bonds are susceptible to attack by a range of chemical agents such as oxidizing agents. Oxidation is one of the more familiar types of chemical reactions (burning fuels and rusting iron are examples) and involves the removal of electrons from one compound, that which is oxidized, by another, called the oxidizing agent. We will discuss oxidation reactions on many occasions down the road, but for now this brief description serves our needs. Gaseous oxygen, as you might surmise from the name, is an excellent oxidizing agent, and alkenes are far more susceptible to attack by oxygen and other oxidizing agents than are alkanes. This is why unsaturated fats tend to go rancid – their double bonds react slowly with oxygen to form foul smelling byproducts. Organisms combat this tendency by employing antioxidants, such as vitamin E, to suppress such reactions. Processed food companies, seeking longer shelf-lives for their products, use a different strategy. Rather than protecting the double bonds from attack, they get rid of them entirely. Specifically, they sometimes chemically modify unsaturated fats by adding hydrogen across their double bonds (see below for further explanation of what this term means) in a process called hydrogenation (Figure 1-33a). In other words, the double bond between the carbon atoms is converted to a single bond as two new carbon-hydrogen bonds are made. This process yields saturated fats in which all double bonds between carbon atoms are converted to single bonds by reactions that are essentially the reverse of that shown in Figure 1-25. The saturated fats that result have longer shelf-lives because they resist attack by oxygen, although there is some controversy concerning their effects on cardiovascular health.[19]
Figure 1-33. Examples of addition reactions: a) addition of H2 to but-1-ene yields butane; in this case an unsaturated hydrocarbon is converted to a saturated one, and; b) addition of water to but-1-ene yields, in this example, butan-1-ol; depending on the reactions conditions employed, one can preferentially form either butan-1-ol or butan-2-ol from this starting material.
Hydrogenation is a specific example of an addition reaction, a general reaction type that alkenes can undergo. In these reactions, a small molecule (or sometimes not so small molecule) “adds” across the atoms connected by a double bond, that is, one fragment of the small molecule adds to one carbon of the double bond and a second fragment adds to the other. In hydrogenation, the small molecule is H2 and both fragments are hydrogen atoms, each of which forms a new carbon-hydrogen bond at the expense of the original hydrogen-hydrogen single bond and one of the two bonds that make up the carbon double bond, leaving those two carbons still connected by a single bond. This type of reaction represents one of the most important classes of reactions in organic chemistry - we will encounter many variations on the theme as many other “small molecules” can likewise be added across double bonds. A particularly important molecule that can do so is water, wherein a hydrogen atom adds to one carbon and the remaining fragment, the hydroxyl, adds to the other (Figure 1-33b), making an alcohol.
Addition reactions provide an entry point for a host of reactions that can modify the structures of alkenes resulting in compounds with very different properties. We conclude this chapter with a brief example of one such series of reactions (Figure 1-34) Consider the but-1-ene case mentioned just above. A relatively simple reaction can take this gaseous hydrocarbon that is virtually insoluble in water to a relatively pleasant smelling liquid that is very water soluble. A follow up reaction, with an oxidizing agent (the details of which need not concern us at this point) can convert an alcohol to a carboxylic acid, converts our alcohol to butanoic acid which, far from having a mild and not unpleasant odor, smells intensely of vomit.
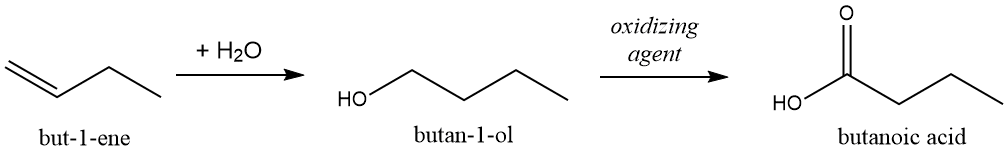
Figure 1-34 A simple reaction sequence that illustrates how functional groups can be transformed, giving rise to new compounds with distinctly different properties from those of the original starting materials.
The material in this rather large section was intended to provide an overview of a few important functional groups and to illustrate the bewildering number of permutations that a few relatively simple structural patterns can take on. Organic chemistry is a huge field because so many possibilities exist when it comes to assembling various functional groups in combination with each other on otherwise inert carbon-hydrogen frameworks. Your task is not to memorize any of it, but to see underlying principles and understand them, allowing you to see the logic of so much behind the chemistry of everyday life.
Footnotes and References.
[17] Unlike a string of beads, however, alkanes tend to assume a particular shape, namely the one that is the most stable. The flexibility arises because there is not much holding that shape in a fixed position as the molecule constantly bumps into its neighbors. You can therefore look at its structure as being flexible, albeit with a bias toward a particular form: left to its own devices, it will revert back to its most stable shape.
[18] This is a bit oversimplified, as double bonds actually consist of two distinctly different types of bonds, one of which is essentially the same type of bonding seen in single bonds; it is the electrons shared in the bond type that is unique to double bonds that are the less tightly held.
[19] A recent study on this topic suggests that health risks of saturated fats are not greater than unsaturated fats. See https://academic.oup.com/eurjpc/advance-article-abstract/doi/10.1093/eurjpc/zwac194/6691821?login=false