1.6B: Carboxylic Acids
- Page ID
- 424831
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carboxylic Acids. As is true with the word “alcohol”, the term “acid” is widely used in common parlance; it can be used to describe materials that are corrosive, or those with a sour or pungent taste. When we discuss the essential ideas of acid/base chemistry in Chapter 5, we’ll discuss acids in greater detail. There are actually many different types of acids but they all do one thing that gives rise to the acidic properties just mentioned: they react with water to form the hydronium ion, H3O+ (this is essentially a water molecule with an extra hydrogen atom and a +1 charge [16]; we’ll discuss ions in detail soon and the details of the reaction need not concern us at the moment). For now we want to introduce a certain type of acid, carboxylic acids, for two reasons: 1) it illustrates another important functional group, and 2) it further illustrates how the structure of molecules impacts their chemical and physical properties.
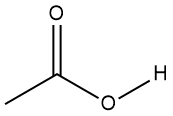
Carboxylic acids are compounds that have an -OH group immediately adjacent to a carbon-oxygen double bond, called a carbonyl group (Figure 1-24). Unlike alcohols, which don’t chemically react with water to a significant extent, the presence of the carbonyl group enhances the reaction with water by orders of magnitude. So, when looking at molecular structures to determine what functional groups are present, don’t make the mistake of concluding that something with a hydroxyl group is necessarily an alcohol: if it is adjacent to a C=O, it is a carboxylic acid.

Figure 1-24. The generic structures of carboxylic acids (left) and alcohols (right); recall that R can be any alkyl or other organic substituent.
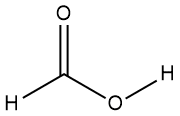
There are many examples of carboxylic acids in Nature, including the linoleic acid found in Burt’s Bees sunscreen. Digestion of plant and animal fats and oils gives rise to several commonly encountered carboxylic acids. These typically have long carbon chains, but there are several relatively familiar carboxylic acids that have short carbon chains. By far the most common of these is acetic acid (pronounced uh-CEE-tick), the compound responsible for the taste and aroma of vinegar. This acid and two other simple examples are shown in Table 1-6 along with some of their properties.
|
Table 1-6: Examples of Simple Carboxylic Acids (RCOOH) |
|||
|
R = |
-H |
-CH3 |
-CH2CH3 |
|
Structural Formula |
|
|
|
|
Line Diagram |
 |
|
|
|
IUPAC Name |
formic acid* |
acetic acid* |
propanoic acid |
|
Other Systematic Name |
methanoic acid |
ethanoic acid |
propionic acid |
|
Boiling Point |
101°C |
118°C |
141°C |
|
Odor |
sharp, pungent |
vinegar |
unpleasant, rancid |
|
* Surprisingly, the IUPAC preferred names for these two acids are not systematic names. See text. |
|||
As with alcohols, the presence of the carboxylic acid functional group (sometimes abbreviated as -COOH) bestows properties to these compounds that are quite different from simple alkanes. (Speculate about why that might be.) The boiling points of these compounds are even higher than those for alcohols with the same number of carbon atoms. Carboxylic acids also show a trend of decreasing solubility in what as the carbon chain increases in length, for precisely the same reason that the same effect was seen in alcohols. The three acids shown in Table 1-6 are miscible with water at room temperature, but if the series is extended a bit, that is no longer true. For example, only about 50 grams of pentanoic acid (C4H9COOH, also known as valeric acid), can dissolve in 1 liter of water at room temperature.
IUPAC has developed a fairly simple systematic naming system for carboxylic acids. For all but the simplest of carboxylic acids, the root of the name is the same as that for the alkane with the same number of carbon atoms, but the -ane suffix for the alkane is replaced by -anoic acid. For example, propane is the alkane having three carbon atoms, and propanoic acid is the carboxylic acid with three carbon atoms. Pentanoic acid acid is the name for the carboxylic acid with a five-carbon backbones, hexanoic acid has six, etc. The only exceptions to this are the two smallest carboxylic acids. According to the IUPAC naming system, one would expect these two acids to be named methanoic and ethanoic acids. Instead, because these two compounds have such deeply entrenched common names, those have been retained by IUPAC. The preferred name for methanoic acid is actually formic acid, named after the Latin word for ants (formic acid is a chief component in certain ant venoms), and that for ethanoic acid is acetic acid, named for the Latin word for vinegar. Other common names are still widely used too, but are not the IUPAC-preferred terms. One final colorful example: butanoic acid, which smells of vomit, has the common name butyric acid (from the Latin for butter, in which butyric acid is formed when it turns rancid); it is also a minor component in parmesan cheese, where it definitely makes its presence known!
Nomenclature: Carboxylic Acids
Naming carboxylic acids is a bit easier than alcohols because the -COOH group is always at the end of a carbon chain (why is that?). This is defined as the first position along the chain. As such, some of the ambiguity about how to number the carbon chains is eliminated. You name the rest of the molecule as you would any other alkane (provided there are no other functional groups present).
 To illustrate we’ll name the molecule at right, which has a slightly modify the carbon backbone than the one we used in the alcohol example earlier. We’ll make the molecule a carboxylic acid by replacing the methyl group that was on carbon 2 with a carbonyl group. This shortens the chain by one carbon atom, but otherwise the backbone is the same. To name the molecule, you:
To illustrate we’ll name the molecule at right, which has a slightly modify the carbon backbone than the one we used in the alcohol example earlier. We’ll make the molecule a carboxylic acid by replacing the methyl group that was on carbon 2 with a carbonyl group. This shortens the chain by one carbon atom, but otherwise the backbone is the same. To name the molecule, you:-
Identify the longest chain that includes the -COOH functional group; the carbon in this functional group is defined as the first carbon of the chain. Recall that when there are two different paths that are equal in length, the priority is given to the path that has the greatest number of substituents.
-
The name of the carboxylic acid ends with the prefix identifying the length of the chain and the suffix -anoic acid; in the above case the name would end with pentanoic acid.
-
The alkyl substituents on the main chain are named and numbered in the usual way; in this case there is a methyl group on carbon 4 and a propyl group on carbon two.
-
Place the substituent names alphabetically and include the corresponding locants.
The correct name for the structure shown is 4-methyl-2-propylpentanoic acid.
Problem 1-18. Provide IUPAC names for the following carboxylic acids.
|
a)
|
b) |
c)
|
Selected Answer:
c) 2-tert-butylpentanoic acid
Problem 1-19. Draw line diagrams that correspond to the following carboxylic acids:
a) 2,3-diethylpentanoic acid
b) 2-propylpentanoic acid
c) 2-isopropyl-3-methylbutanoic acid
d) 2-(butan-2-yl)pentanoic acid
Problem 1-20. Consider all of the possible carboxylic acids having five total carbon atoms and only one carboxylic acid functional group. What is the molecular formula for these compounds? How many isomers will exist? Draw and name them all.
Footnotes and References
[16] A definition we'll return to at length on multiple occasions: an ion is an atom or molecule that is not electrically neutral; they can bears either a positive or negative charge.