1.6A: Alcohols
- Page ID
- 424838
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alcohols. We’ll begin our discussion of functional groups with what is perhaps the one most familiar to non-chemists: alcohols. While the term “alcohol” is commonly used to refer to alcoholic beverages, in a chemical context it is actually a broader term, referring to compounds containing a hydroxyl group, which consists of one atom of oxygen and one of hydrogen and written as “-OH”. The dash in “-OH” does not indicate a charge or have other significance other than to indicate that this is where the -OH is connected to a larger organic structure in the same way that alkyl groups like methyl do. You’ve probably heard of rubbing alcohol, which is another name for isopropyl alcohol (which is yet another name for the more systematic, but clumsy, propan-2-ol; more on naming alcohols later in this section). As the name implies, the -OH group in isopropyl alcohol is attached to an isopropyl group (Table 1-4). Another familiar alcohol is what hardware stores often sell as “wood alcohol”, a commonly used solvent; wood alcohol is another name for methyl alcohol, or methanol. The alcohol that is found in alcoholic beverages is ethanol, so named because it is formally derived from the two-carbon alkane, ethane.
| Table 1-4: Examples of Familiar Alcohols (ROH) | |||
| R= | -CH3 | -CH2-CH3 | -CH(CH3)2 |
| structural formula | |||
| line diagram | |||
| common names | wood alcohol | grain alcohol | rubbing alcohol |
| IUPAC-recommended names | methanol | ethanol | propan-2-ol |
| other systematic names | methyl alcohol | ethyl alcohol | isopropanol, isopropyl alcohol |
| boiling point (°C) | 64.7 | 78.2 | 82.6 |
In theory any alkane can be converted to an alcohol by replacing a hydrogen atom with a hydroxyl substituent and, depending on the alkane you start with, it is possible to generate multiple isomers by replacing different hydrogen atoms. Consider propane, C3H8. If you replace one hydrogen with a hydroxyl group you will get a structure with the formula C3H7OH. One might ask at this point, if there are 8 hydrogen atoms that can be replaced by a hydroxyl, are there eight possible isomers of the resulting alcohol? No. The reason is that some of these hydrogen atoms are “equivalent”, meaning that they are for all intents and purposes identical to each other. Replacing equivalent hydrogen atoms gives rise to identical products. In this case, there are two sets of “equivalent” hydrogen atoms: a group of six located on the two terminal methyl groups, and a group of two located on the central carbon atom (Figure 1-22). How would you recognize that they are equivalent? There are several ways, one of which is to examine the various products you get when doing the hydroxyl-for-hydrogen swap.[11] If you replace any of the six red hydrogen atoms in Figure 1-22, the result is an alcohol in which the hydroxyl is located on a terminal carbon of a three-carbon chain: there is no discernable difference between them, even if, on paper, there might be 6 different ways of drawing them. This is an example of how "flattening" a three-dimensional structure onto a two-dimensional surface can be misleading. Likewise, if you replace either of the two hydrogen atoms labeled in blue, the result is that the hydroxyl substituent is on the central carbon of a three-carbon chain. There are therefore only two isomers of C3H7OH that exist [12]: one with the hydroxyl on a terminal carbon atom (called propan-1-ol), and one with it on the central carbon atom (called propan-2-ol). This approach, by the way, is in no way restricted to thinking about isomers of alcohols. It doesn't matter what substituent we might want to replace a hydrogen with, the result is the same: only two isomeric forms of the new compound are possible. For example, if we wanted to replace a hydrogen atom with a methyl substituent, replacing any of the red hydrogen atoms in Figure 1-22 would yield butane, while replacing a blue hydrogen would result in 2-methylpropane.

Figure 1-22. Propane has two sets of equivalent hydrogen atoms: the 6 on the two terminal carbon atoms (red), and the 2 on the central carbon (blue), so only two isomeric alcohols can be formed by replacing any one of the hydrogen atoms with a hydroxyl. Replacing one of the 6 hydrogen atoms of the methyl groups results in propan-1-ol (bottom left), while replacing either of the two hydrogen atoms on the central carbon results in propan-2-ol (bottom right).
In contrast to the above, consider the simplest alkane, CH4: it doesn’t matter which hydrogen you replace with a hydroxyl, you will get the same product, methanol, meaning that all four hydrogen atoms of CH4 are equivalent. The same is true with all six hydrogen atoms of ethane, C2H6; replacing any one of these six gives rise to the same structure: ethanol. For alkanes with three or more carbon atoms, however, different isomers are usually (but not always!) possible when you replace a hydrogen with a hydroxyl substituent.
Problem 1-13. Identify how many sets of equivalent hydrogen atoms exist for the following alkanes.
a) butane
b) 2-methylpropane
c) 2-methylbutane
d) pentane
e) 2,4-dimethylpentane
f) 2,2-dimethylbutane
Problem 1-14. For each of the above compounds, determine how many isomers can be formed by replacing one hydrogen with a hydroxyl substituent.
Returning to the information in Table 1-4, we included the boiling points to illustrate the stark differences between alcohols and alkanes. Specifically, all of these alcohols are liquids at room temperature while all of the parent alkanes – methane, ethane and propane – are gasses, meaning their boiling points are lower than room temperature. Why would such a seemingly simple structural change cause the boiling point to increase so much? We will discuss the above question in detail when we cover intermolecular forces in Chapter 7 but, for now, consider what it means for something to boil. First, it is important to note that all molecules are attracted to each other, sort of like magnets. And just as some magnets are stronger than others, meaning that some are easier to pull apart, different molecules have different types of attractive forces that have different strengths. To boil a liquid means that there is enough energy (in the form of thermal energy provided by heat) that the molecules have enough energy to overcome the attraction they have for each other, sort of like a rocket having enough energy to pull away from the gravitational attraction of the Earth. This is what happens when any liquid boils. The stronger the attractive forces that exist between molecules, the more energy is required to overcome the attraction and, consequently, the higher the boiling point because it requires a greater temperatures to attain the required thermal energy.
Based on the above logic, we can conclude that molecules of methanol are much more strongly attracted to each other than are molecules of methane. Why would this be so? Recall from Figure 1-13 that water molecules are polar because they have O-H bonds (the charge is polarized, meaning unequally distributed through the molecule). Methanol, because it is an alcohol, also has an O-H bond and is “water-like” in some important ways. As is true with water, the hydrogen attached to the oxygen in methanol is somewhat positively charged, and the oxygen is somewhat negatively charged. This gives rise to an electrostatic attractive force between molecules and this mutual attraction of methanol molecules for one another is manifested in its boiling point being about 225°C higher than its parent alkane.
 |
 |
 |
 |
|
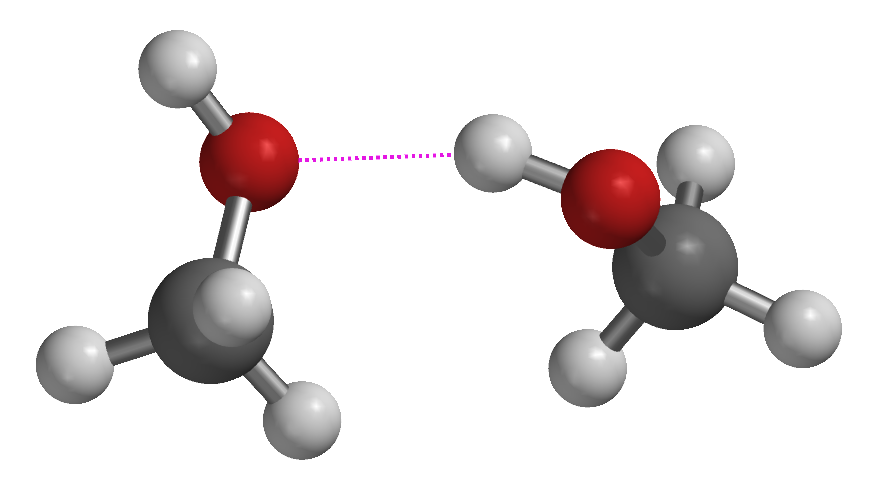
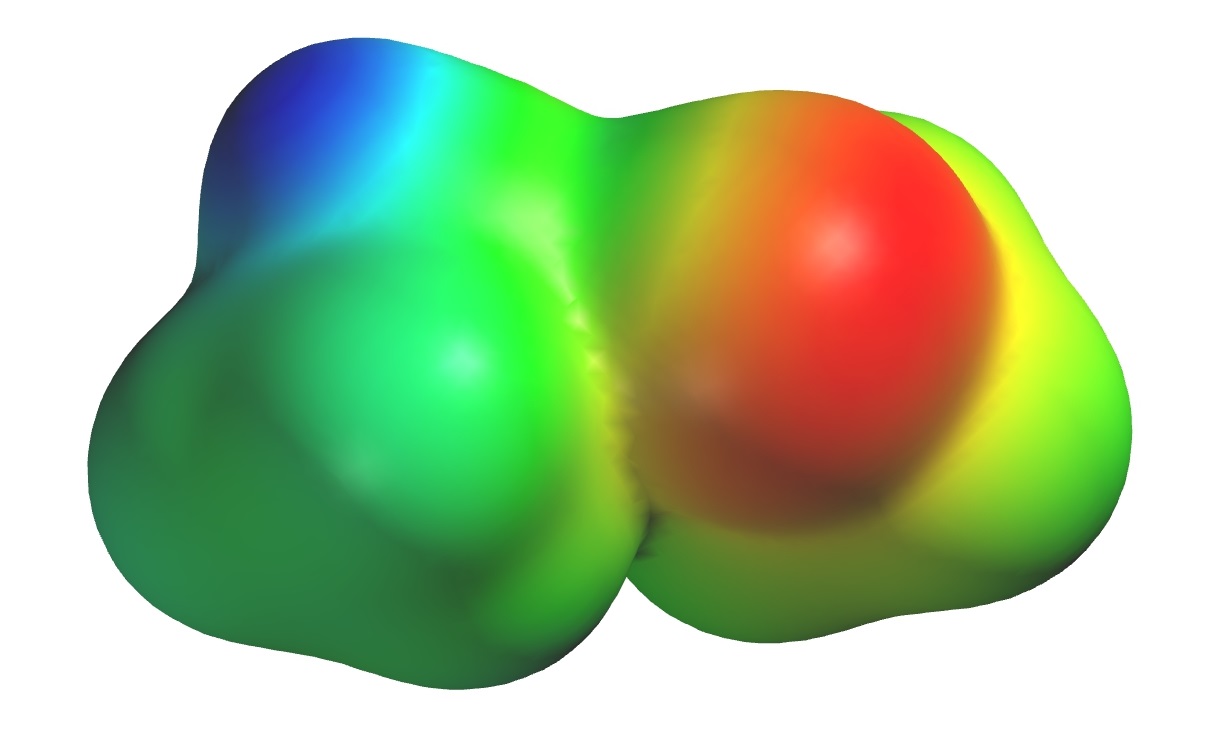
Figure 1-23. Comparison of how pairs of methane (top) and methanol (bottom) molecules interact. The left column shows ball and stick models and approximates the most stable distance between two molecules; note that the methanol molecules are much closer together, reflecting the stronger attraction between them (the dotted line between the oxygen on the left and the hydrogen on the right indicates where the attraction is greatest). The right side shows the esp maps for the same pairs of molecules. The methane molecules have virtually no charge imbalance (the green color of the esp maps on the right hand side illustrate this) at their surface and therefore have no appreciable positive/negative attractive forces; they do not require a high temperature to be separated and consequently methane has a very low boiling point. The charge imbalance of methanol (blue indicates positive charge, red indicates negative) gives rise to a strong electrostatic attraction, bringing the molecules closer together and resulting in its much higher boiling point. |
|
Figure 1-23 shows pairs of methanol and methane molecules at their “optimum” distances apart (where they are most stable). Methane molecules have very little charge separation (the green color indicates that the surface is nearly electrically neutral) and therefore do not have a strong attraction for one another. In contrast, two methanol molecules approach each other much more closely because the negative oxygen on one attracts the positive oxygen on the other. The dashed line emphasizes the interaction. The esp maps actually show that the molecules of methanol are partially “fused” because of the attraction between them, just as two magnets stick together closely, but two marbles don’t.
The above discussion about alcohols focused primarily on the structure of alcohols and their physical properties, but what can we say about their chemical nature? Can we describe alcohols in a manner analogous to how we described alkanes? Alcohols are certainly more reactive than alkanes, and have a wider range of chemical properties. Like alkanes, alcohols are flammable (alcohol burners used to be quite common). But unlike alkanes, alcohols exhibit a very wide range of solubilities in water. Small alcohols, such as methanol, ethanol and propan-2-ol, are miscible with water, meaning they can be mixed in water in any proportion without separating; they have, in essence, infinite solubility in water. Larger alcohols are less soluble in water. For example, neither butan-1-ol or butan-2-ol are miscible with water, but they are nevertheless quite soluble compared to even larger alcohols. For example you can dissolve 73 g of butan-1-ol in 1 liter of water (at room temperature), meaning that if you add up to that amount, the result will be a homogeneous solution where the water and butan-1-ol molecules are completed mixed at the molecular level. If one tried to add more than 73 g per liter, however, some of the alcohol would separate from the water, forming a separate phase, like oil floating on water. Table 1-5 presents the solubilities of alcohols having the formula CH3(CH2)xOH, that is, a series of alcohols derived from simple unbranched alkanes that have the hydroxyl group on a terminal carbon [13]. What pattern emerges and how can we explain it?
Table 1-5. Structures, Names and Solubilities of Alcohols in the Series CH3(CH2)xOH
| Name | Formula | Line Diagram | Solubility (g/L H2O) |
| methanol | CH3OH |  |
miscible |
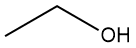
| ethanol | CH3CH2OH (or C2H5OH) |
 |
miscible |
| propan-1-ol | CH3(CH)2OH (or C3H7OH) |
 |
miscible |
| butan-1-ol | CH3(CH)3OH (or C4H9OH) |
 |
73 |
| pentan-1-ol | CH3(CH)4OH (or C5H11OH) |
 |
22 |
| hexan-1-ol | CH3(CH)5OH (or C6H13OH) |
 |
5.9 |
| heptan-1-ol | CH3(CH)6OH (or C7H15OH) |
 |
1.0 |
| octan-1-ol | CH3(CH)7OH (or C8H17OH) |
 |
0.30 |
| nonan-1-ol | CH3(CH)8OH (or C9H19OH) |
 |
0.13 |
| decan-1-ol | CH3(CH)9OH (or C10H21OH) |
 |
0.037 |
Clearly, the longer the carbon chain the lower the alcohol’s solubility in water. The properties of these molecules become increasingly similar to alkanes as the chains increase in length, which makes sense because the portion of the molecule that is polar and hydrophilic becomes relatively smaller as the number of carbon atoms increases. On a conceptual level, these compounds have two structural features with very different, and opposing, characteristics: the hydrophilic hydroxyl group, and the hydrophobic alkyl group. The larger the hydrophobic group, the more the molecule takes on that characteristic. The smaller the hydrophobic group, the more the molecule takes on the “water-like” character of the hydroxyl group. And the effect is not subtle!
The above is a good example of how the properties of molecules can be “tuned”; we can make compounds more or less soluble in water by controlling the length of the alkyl chain. Nature figured this out a long time ago and decided to make cell membranes out of molecules with hydrophilic "heads" and long hydrophobic "tails" because they resist dissolving in water and therefore provide structural stability in an aqueous environment. [14]
Turning our attention to the nomenclature of alcohols, the IUPAC names of the alcohols are listed in Tables 1-4 and 1-5. For the two simplest alcohols, the –ane suffix of the parent alkane is simply replaced by the an –anol suffix. Hence methane becomes methanol when a hydroxyl replaces a hydrogen, and ethane becomes ethanol. When different isomers can exist, the location of the -OH group is specified by a locant that precedes the -ol portion of the suffix, with the lowest number possible being used. For example, if you have a molecule consisting of a five carbon chain, with the only substituent being a hydroxyl on the second carbon, its IUPAC name would be pentan-2-ol. [15]
Nomenclature: Alcohols
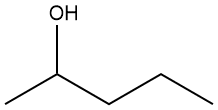
As you might expect, additional subtleties arise when naming alcohols more complex than those in Table 1-4 or 1-5; guidelines for naming such compounds are presented below. While the general approach is similar to that used to name alkanes, there are some noteworthy differences. We’ll use the example at right to illustrate. 
-
Identify the longest chain that includes the carbon to which the hydroxyl is connected. This is an important difference from how we named alkanes. The longest carbon chain in the structure above has seven carbons but it doesn’t include the carbon bearing the hydroxyl group. The longest chain that does include the carbon bound to the hydroxyl has only six carbons.
-
Identify the position along the chain where the hydroxyl is located. Number the chain so that the hydroxyl has the smallest possible locant. In this case, the hydroxyl is on the second carbon of the longest chain (not the fifth) so, therefore, the name will end in hexan-2-ol.
-
Identify the alkyl substituents on the main chain. This structure presents some interesting ambiguities in that regard. Specifically, at the third carbon, there are two alkyl substituents, each of which could be the “main chain” because they both give chains that are six carbons long. What to do? Recall from the guidelines previously presented for alkanes, define as the main chain that pathway that has the greatest number of substituents. In this structure, that means that the main chain goes left at carbon three because that pathway has a propyl group at carbon 3 and a methyl at carbon 5.
-
Append the substituent names (alphabetically) with appropriate locants to the base name we've already determined (hexan-2-ol). The name is: 5-methyl-3-propylhexan-2-ol [not 3-(2-methylpropyl)hexan-2-ol)].
Problem 1-15. Provide IUPAC names for the following alcohols.
|
a) |
c)
|
e) |
|
b) |
d)
|
f) |
- Selected Answers.
-
c) pentan-2-ol (older systematic name: 2-pentanol)
d) 2,4-dimethylpentan-3-ol (older systematic name: 2,4-dimethyl-3-pentanol )
Problem 1-16. Draw line diagrams and provide IUPAC names for all alcohols having the formula C5H11OH.
Problem 1-17. Draw and name as many alcoholic isomers of hexan-1-ol that you can. (An alcoholic isomer is an isomer that has an -OH group). Hint: There are 17 alcoholic isomers with the formula C6H13OH
Footnotes and References
[11] A more elegant way of determining whether or not atoms in a molecule are equivalent is through the use of symmetry: if atoms can "reflect" into each other, or "rotate" into each other's positions, they are equivalent. We will develop the idea of molecular symmetry more when we discuss the three-dimensional structure of molecules.
[12] To be more accurate, only two alcohols have the formula C3H7OH. If you consider other functional groups, another isomer that has the formula C3H8O also exists. It is an ether, a functional group we introduce in Chapter 4.
[13] Alcohols with the hydroxyl group on terminal carbon atoms are sometimes referred to as “primary alcohols”. This term emphasizes that the carbon that is bonded to the hydroxyl group is itself bonded to only one other carbon atom. Alcohols like propan-2-ol, with the hydroxyl on a carbon connected to two other carbons, are secondary alcohols. Tertiary alcohols also exist; these have the hydroxyl group bound to a carbon that is itself bonded to three other carbon atoms. These terms become more important when considering the chemical reactivity of alcohols, which can be quite different for primary, secondary and tertiary alcohols.
[14] We should note that cell membranes are not composed of simple alcohols, however, but the compounds that are “close relatives” to alcohols and the idea reflected in the data in the above table is certainly relevant to understanding how cell membranes work.
[15] This compound would also go by another systematic name, 3-pentanol. This alternative naming scheme has been replaced by IUPAC but is still widely used by chemists, partly because the name flows better off the tongue: 3-pentanol sounds more like actual words than pentan-3-ol does, at least to this human. In this text, we will emphasize the IUPAC system because that is what is often preferred by journals.