Anti-Cancer Drugs II
- Page ID
- 411
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydroxyurea
Hydroxyurea blocks an enzyme which converts the cytosine nucleotide into the deoxy derivative. In addition, DNA synthesis is further inhibited because hydroxyurea blocks the incorporation of the thymidine nucleotide into the DNA strand.
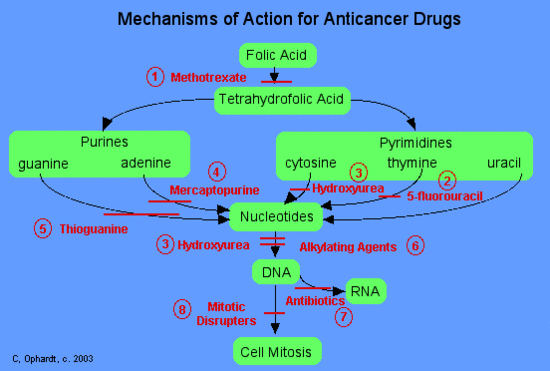
Mercaptopurine
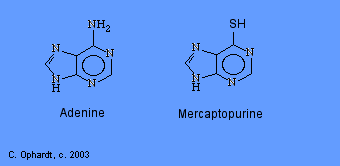
Mercaptopurine, a chemical analog of the purine adenine, inhibits the biosynthesis of adenine nucleotides by acting as an antimetabolite. In the body, 6-MP is converted to the corresponding ribonucleotide. 6-MP ribonucleotide is a potent inhibitor of the conversion of a compound called inosinic acid to adenine Without adenine, DNA cannot be synthesized. 6-MP also works by being incorporated into nucleic acids as thioguanosine, rendering the resulting nucleic acids (DNA, RNA) unable to direct proper protein synthesis.
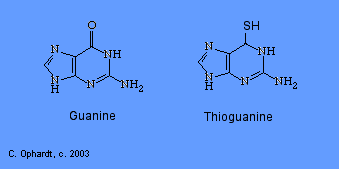
Thioguanine
Thioguanine is an antimetabolite in the synthesis of guanine nucleotides.
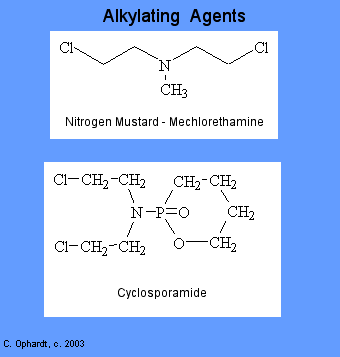
Alkylating Agents
Alkylating agents involve reactions with guanine in DNA. These drugs add methyl or other alkyl groups onto molecules where they do not belong. This in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA.
- In the first mechanism an alkylating agent attaches alkyl groups to DNA bases. This alteration results in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases.
- A second mechanism by which alkylating agents cause DNA damage is the formation of cross-bridges, bonds between atoms in the DNA. In this process, two bases are linked together by an alkylating agent that has two DNA binding sites. Cross-linking prevents DNA from being separated for synthesis or transcription.
- The third mechanism of action of alkylating agents causes the mispairing of the nucleotides leading to mutations.
There are six groups of alkylating agents: nitrogen mustards; ethylenimes; alkylsulfonates; triazenes; piperazines; and nitrosureas. Cyclosporamide is a classical example of the role of the host metabolism in the activation of an alkylating agent and is one or the most widely used agents of this class. It was hoped that the cancer cells might posses enzymes capable of accomplishing the cleavage, thus resulting in the selective production of an activated nitrogen mustard in the malignant cells. Compare the top and bottom structures in the graphic on the left.
Antibiotics
A number of antibiotics such as anthracyclines, dactinomycin, bleomycin, adriamycin, mithramycin, bind to DNA and inactivate it. Thus the synthesis of RNA is prevented. General properties of these drugs include: interaction with DNA in a variety of different ways including intercalation (squeezing between the base pairs), DNA strand breakage and inhibition with the enzyme topoisomerase II. Most of these compounds have been isolated from natural sources and antibiotics. However, they lack the specificity of the antimicrobial antibiotics and thus produce significant toxicity.
The anthracyclines are among the most important antitumor drugs available. Doxorubicin is widely used for the treatment of several solid tumors while daunorubicin and idarubicin are used exclusively for the treatment of leukemia. These agents have a number of important effects including: intercalating (squeezing between the base pairs) with DNA affecting many functions of the DNA including DNA and RNA synthesis. Breakage of the DNA strand can also occur by inhibition of the enzyme topoisomerase II.
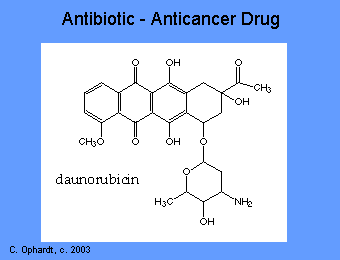
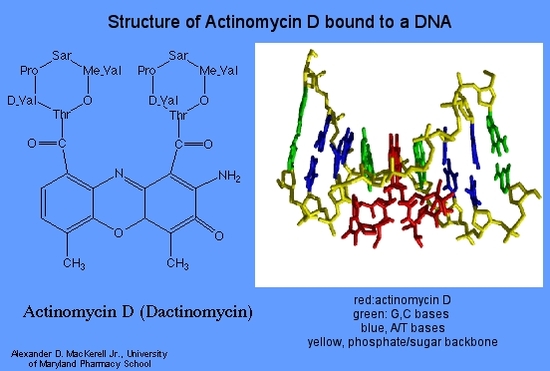
Dactinomycin (Actinomycin D)
At low concentrations dactinomycin inhibits DNA directed RNA synthesis and at higher concentrations DNA synthesis is also inhibited. All types of RNA are affected, but ribosomal RNA is more sensitive. Dactinomycin binds to double stranded DNA , permitting RNA chain initiation but blocking chain elongation. Binding to the DNA depends on the presence of guanine.
Mitotic Disrupters
Plant alkaloids like vincristine prevent cell division, or mitosis. There are several phases of mitosis, one of which is the metaphase. During metaphase, the cell pulls duplicated DNA chromosomes to either side of the parent cell in structures called "spindles". These spindles ensure that each new cell gets a full set of DNA. Spindles are microtubular fibers formed with the help of the protein "tubulin". Vincristine binds to tubulin, thus preventing the formation of spindles and cell division.
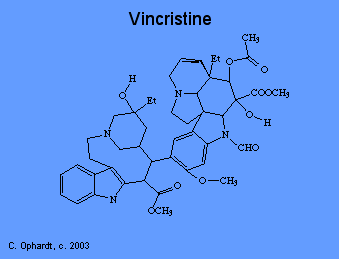
Taxol
Paclitaxel (taxol) was first isolated from the from the bark of the Pacific Yew (Taxus brevifolia). Docetaxel is a more potent analog that is produced semisynthetically. In contrast to other microtubule antagonists, taxol disrupts the equilibrium between free tubulin and mircrotubules by shifting it in the direction of assembly, rather than disassembly. As a result, taxol treatment causes both the stabilization of microtubules and the formation of abnormal bundles of microtubules. The net effect is still the disruption of mitosis.
Mechanism of Intercalating Agents
Intercalating agents wedge between bases along the DNA. The intercalated drug molecules affect the structure of the DNA, preventing polymerase and other DNA binding proteins from functioning properly. The result is prevention of DNA synthesis, inhibition of transcription and induction of mutations. Examples include: Carboplatin and Cisplatin.
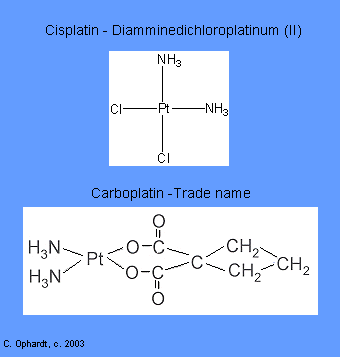
These related drugs covalently bind to DNA with preferential binding to the N-7 position of guanine and adenine. They are able to bind to two different sites on DNA producing cross-links, either intrastrand (within the same DNA molecule which results in inhibition of DNA synthesis and transcription.
Contributors
Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook

