Introduction
- Page ID
- 359801
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What Is Organic Chemistry and Why Is It Important?
On a lovely Saturday afternoon in April, you are relaxing in a garden whilst enjoying a hot cup of coffee. Colourful spring blossoms lace the air with a pleasant aroma, and the green grass, warm sunshine and rich espresso make the afternoon a charming occasion.
Your mind begins to drift as you contemplate the combination of scents, colours and tastes that surround you in this moment, and how they make up the human experience’s unique and fascinating complexities.
If you have ever wondered about the origins of nature’s vibrant hues or the reasonings behind the alluring flavour of coffee, you would be able to find every answer within the elaborate spectrum of knowledge in the study of Organic Chemistry.
Organic chemistry is the chemistry of compounds containing the carbon element: the common element of all living organisms. Anthocyanins are the pigments that give flowers their various colours, chlorophyll is responsible for the green shades of grass is involved in the photosynthesis process of plants, and caffeine is what makes coffee function the way that it does. All these substances contain carbon, and they are all organic compounds.
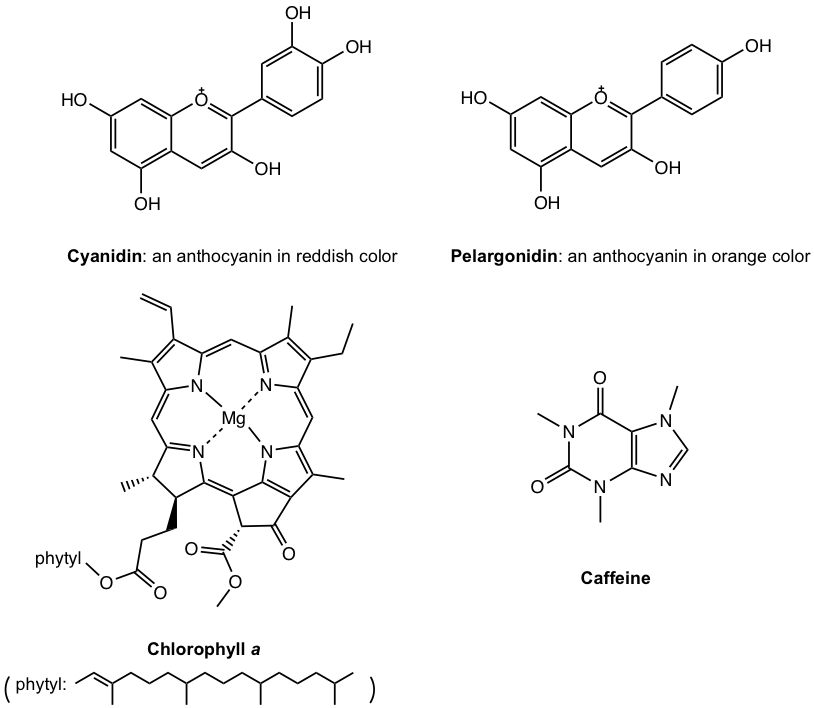
The root of the term organic dates back from over two hundred years ago, when its original meaning did not even involve the element of carbon. The word organic was first introduced in 1807 by Jöns Jakob Berzelius, a Swedish chemist, and was used to refer to compounds derived from living organisms. It was once believed that organic compounds could only be obtained directly from nature as they contained a mystical essence of life known as “vita force”, therefore making it impossible to create organic compounds artificially. This theory was shattered by a famous experiment conducted by German chemist Friedrich Wohler in 1828. In his experiment, Wohler successfully synthesized the crystal urea by heating ammonia and cyanic acid together. The synthesis of urea marked a new era in the history of organic chemistry, not only redefining the term organic, but also rerouting organic chemistry into a completely new scientific discipline. The contemporary definition of organic, being carbon-containing compounds, is now the scientific way of describing the term. However, it has remained true over the years that organic compounds are essential to every known lifeform, as an abundance of organic molecules constitute all living organisms.
There are two additional notes regarding the modern definition of organic. Firstly, while it is true that organic compounds are those containing the element carbon, it is important to know that not all compounds that contain carbon are organic compounds. For example, calcium carbonate (CaCO3), the primary component in certain rocks and chalk, can never be labelled as organic. Secondly, the “organic” food that is often found in supermarkets refers to the fact that the agricultural products were grown without the use of artificial pesticides, herbicides, or synthetic fertilizers, and has nothing to do with the presence of carbon in their chemical structures. This use of the word organic is possibly derived from the old definition, implying that the products came from nature, without human intervention.
As you may have been able to deduce, organic chemistry can be found in every corner of the world around us. From the food we eat, (the carbohydrates in bread, the protein in meat, the fructose in fruit, and more) to the fabric we wear, (cotton, nylon, polyester) and the fuels that power the technology around us (gasoline, natural gas, coal), the list of organic compounds involved in our lives is endless. An important significance in the application of organic chemistry is its critical role in the development of medicine and pharmaceuticals. The active ingredients found in medicine are most often organic compounds, either isolated from naturally occurring materials or synthesized in a lab. Just a few well-known examples include Aspirin, Tylenol, penicillin, insulin, Warfarin, and Tamiflu. The rapid developments of the pharmaceutical industry, in which organic chemistry has acted as a major driver, have saved millions of lives and has dramatically improved today’s quality of life.
The magic element that is the key to organic chemistry and all living organisms is carbon. What is it about the carbon element that makes it so special? This can mainly be attributed to the special bonding ability that carbon possesses. Carbon atoms can form strong covalent bonds with other carbon atoms in the form of chains and rings, and it also forms strong bonds with other elements such as hydrogen, oxygen, nitrogen, sulfur and more. As a result, the structures of organic compounds are hugely diverse and can be rather complex.
Tips for Studying Organic Chemistry
Learning organic chemistry can be both exciting and challenging. The most commonly misleading learning strategy is the notion that “I can be successful by simply memorizing everything”. While memorization may be necessary at times, it is but a small fraction of what is needed to learning organic chemistry; the more important factor is your understanding. There are many structures, reactions and mechanisms involved in the course, and surface-level memorization will not carry you all the way through. However, if you know the connections between the structures, understand the underlying principles of the reactivity of certain compounds, and can tell the similarities and differences between different mechanisms, you will find that it becomes much easier. A few suggestions for learning include:
- Rewrite your own notes when studying. For example, restate the concepts in your own words, or write a map of the concepts that are related.
- Practice makes perfect. Do as many practice questions as you can, and try to make your own questions to double check your understanding.
- Use molecule model sets for certain topics.
About the Book
Due to the high price barrier, about half of Organic Chemistry students at KPU do not have access to the textbook. This has become a serious issue that significantly affects the learning outcomes for the course. The creation of this open textbook is intended to provide a solution to this problem and help students get success in this course.
The book contains ten chapters, with the contents cover from the basic concepts on chemical bonding, functional group, to stereochemistry, spectroscopy for structure determination (IR and NMR) and organic reactions (nucleophilic substitution, elimination, radical substitution of alkanes, addition and oxidation reactions of alkenes, preparation and reactions of alkynes).
Organic Chemistry is a challenging subject for many students. To help readers understand the concepts more easily, simple and concise languages are intentionally applied in the book. The featured shaded textbox areas are included frequently in the book, where readers can find useful learning tips, reminder of common errors, comparison between similar concepts. To help readers develop problem-solving skills, a small section labelled as “strategy” is usually given for the examples in the book. Readers are encouraged to try solving the problems by themselves with helpful hints provided in the “strategy”, and then compare their work with the detailed solutions provided afterwards. Because of limited time availability, not many practice questions are included in this book. We hope that more questions, particularly questions with different level of difficulties, will be added in the future edition of the book.

