9.10: Sulfur Analogs of Alcohols and Ethers
- Page ID
- 32406
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
-
- write the IUPAC name of a thiol, given its Kekulé, condensed or shorthand structure.
- draw the structure of a thiol, given its IUPAC name.
- write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide.
- write an equation to illustrate the preparation of a thiol by the reaction of thiourea with an alkyl halide.
- write an equation to show the interconversion between thiols and disulfides.
-
- write the name of a sulfide, given its structure.
- draw the structure of a sulfide, given its name.
- write an equation showing how a sulfide may be prepared by the reaction of a thiolate anion on an alkyl halide.
- identify the product from the reaction of a given alkyl halide with a given thiolate anion.
- identify the reagents necessary to prepare a given sulfide.
- write an equation to illustrate the formation of a trialkylsulfonium salt from a sulfide and an alkyl halide.
Note: All of these terms are defined in the “Study Notes,” below.
- disulfide
- mercapto group
- (organic) sulfide
- sulfone
- sulfoxide
- thiol
- thiolate anion
- trialkylsulfonium ion (trialkylsulfonium salt)
The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. However, we have included a short section on these compounds, not for the sake of increasing the amount of material to be digested, but because much of the chemistry of these substances can be predicted from a knowledge of their oxygen-containing analogues.
A thiol is a compound which contains an SH functional group. The SH group itself is called a mercapto group. A disulfide is a compound containing an S-S linkage.
(Organic) sulfides have the structure R-S-R′, and are therefore the sulfur analogues of ethers. The nomenclature of sulfides can be easily understood if one understands the nomenclature of the corresponding ethers. Notice that the term “thio” is also used in inorganic chemistry. For example, SO42− is the sulfate ion; while S2O32−, in which one of the oxygen atoms of a sulfate ion has been replaced by a sulfur atom, is called thiosulfate. Thiolate anions, RS-, are analogous to alkoxy anions, RO-. Thiolate anions are better nucleophiles than are alkoxy anions.
If you have trouble understanding why trialkylsulfonium ions are formed, think of them as being somewhat similar to the hydronium ions that are formed by protonating water:
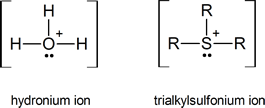
Later we shall see examples of tetraalkylammonium ions, R4N+, which again may be regarded as being similar to hydronium ions.
Sulfoxides and sulfones are obtained by oxidizing organic sulfides. You need not memorize the methods used to carry out these oxidations.
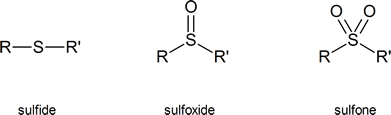
Table 18.1, below, provides a quick comparison of oxygen-containing and sulfur-containing organic compounds.
| Oxygen-containing Compound | Sulfur Analogue |
|---|---|
| ether, R-O-R′ | sulfide, R-S-R′ |
| alkoxy anion, RO- | thiolate anion, RS- |
| alcohol, ROH | thiol, RSH |
| hydroxy group, OH- | mercapto group, SH- |
| peroxide, R-O-O-R′ | disulfide, R-S-S-R′ |
Note that when we name thiols, we include the “e” of the alkane name. Thus, CH3CH2SH is called “ethanethiol,” not “ethanthiol.”
Thiols and sulfides are the "sulfur equivalent" of alcohols and ethers. You can replace the oxygen atom of an alcohol with a sulfur atom to make a thiol; similarly, you can replace the oxygen atom in an ether with S to make the corresponding alkyl sulfide. This is because thiols contain the C-S-H functional group, while sulfides contain the C-S-C group.
Oxidation States of Sulfur Compounds
Oxygen assumes only two oxidation states in its organic compounds (–1 in peroxides and –2 in other compounds). Sulfur, on the other hand, is found in oxidation states ranging from –2 to +6, as shown in the following table (some simple inorganic compounds are displayed in orange).
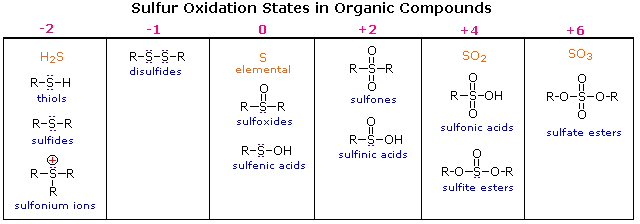
Thiols
Thiols are often called “mercaptans,” a reference to the Latin term mercurium captans (capturing mercury), since the -SH group forms strong bonds with mercury and its ions. Thiols are analogous to alcohols. Thiols are weakly acidic (pKa ~ 10) and are much stronger acids than alcohols (pKa ~ 16). However, thiols usually do not form hydrogen bonds due to the sulfur atom not have sufficient electronegativity. Thiols named using the same rules as alcohols except the parent chain is named as alkane with the suffix -thiol added. As a substituent the -SH group is called a mercapto group.
Thiols are usually prepared by using the hydrosulfide anion (-SH) as a nucleophile in an SN2 reaction with alkyl halides.
One problem with this reaction is that the thiol product can deprotonate and undergo a second SN2 reaction with an additional alkyl halide to produce a sulfide side product. This problem can be solved by using thiourea, (NH2)2C=S, as the nucleophile. The SN2 reaction first produces an alkyl isothiourea salt as an intermediate. This salt is then hydrolyzed to form the thiol by a reaction with aqueous base.
Disulfides
Thiols (R-S-H) can be oxidized to form disulfides (R-S-S-R') through reaction with Br2 or I2. Note, an equivalent oxidation of alcohols (R-O-H) to peroxides (R-O-O-R') is not normally observed. The reasons for this different behavior becomes clear when looking at bond strengths. The S–S single bond in disulfides is nearly twice as strong as the O–O bond in peroxides. Also, the O–H bond in alcohols is more than 25 kcal/mole stronger than the S–H bond in thiols. Thus, thermodynamics favors disulfide formation over peroxide. Disulfides can easily be reduced back to a thiols through reaction with zinc and acid.
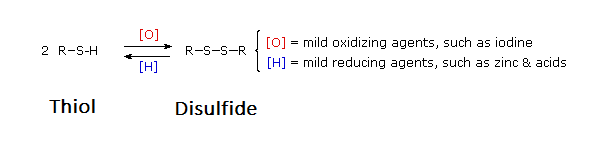
Disulfide (sulfur-sulfur) linkages between two cysteine residues are an integral component of the three-dimensional structure of many proteins. The interconversion between thiols and disulfide groups is a redox reaction: the thiol is the reduced state, and the disulfide is the oxidized state.
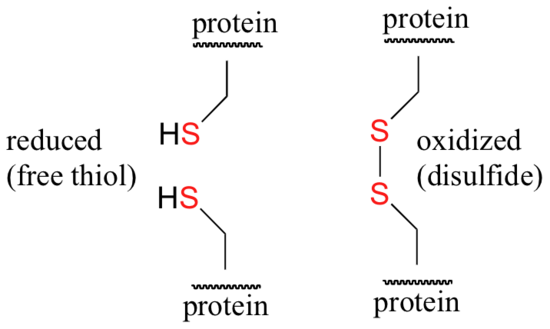
Notice that in the oxidized (disulfide) state, each sulfur atom has lost a bond to hydrogen and gained a bond to a sulfur - this is why the disulfide state is considered to be oxidized relative to the thiol state. The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme. Recall that the important functional group in glutathione is the thiol, highlighted in blue in the figure below. In its reduced (free thiol) form, glutathione is abbreviated 'GSH'.
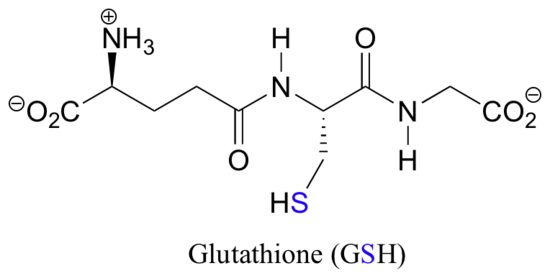
In its oxidized form, glutathione exists as a dimer of two molecules linked by a disulfide group, and is abbreviated 'GSSG'. A new disulfide in a protein forms via a 'disulfide exchange' reaction with GSSG, a process that can be described as a combination of two SN2-like attacks. The end result is that a new cysteine-cysteine disulfide forms at the expense of the disulfide in GSSG.
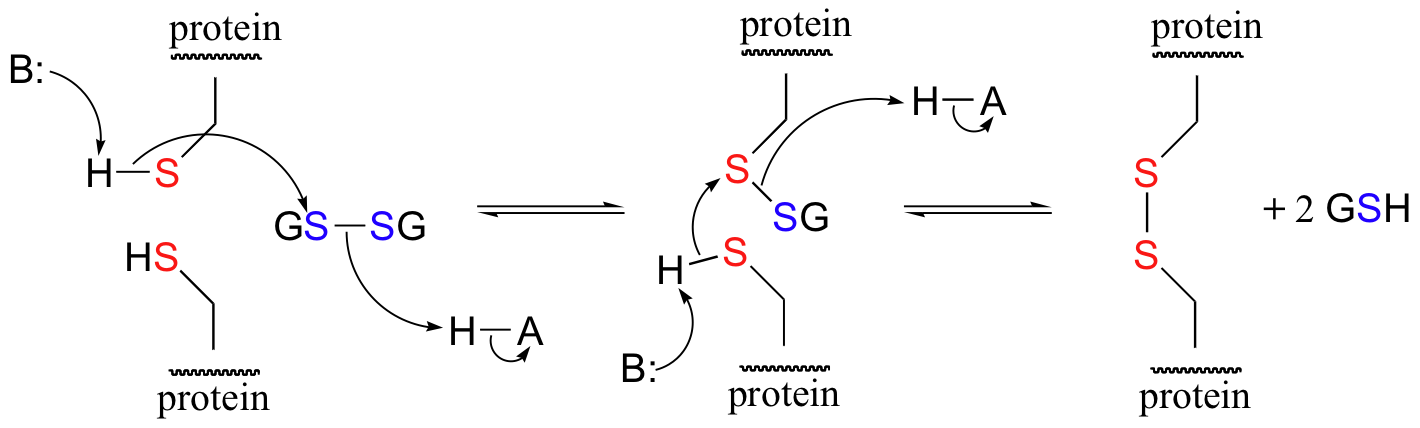
In its reduced (thiol) state, glutathione can reduce disulfides bridges in proteins through the reverse of the above reaction.
Sulfides
Sulfides are named using the same rules as ethers except sulfide is used in the place of ether. For more complex substances, alkylthio is used in place of alkoxy.
Sulfur analogs of ethers are called sulfides. Sulfides are less common than thiols as naturally occurring compounds. However, sulfides—especially disulfides (C-S-S-C)—have important biological functions, mainly in reducing agents (antioxidants).
Since thiols are weakly acidic their conjugate bases, called thiolate ions, can be easily formed through reaction with a strong base such as NaH. Thiolates have proven to be excellent nucleophiles and easily undergo SN2 reactions with primary and secondary alkyl halides to form sulfides. The reaction is analogous to the Williamson ether synthesis previously discussed in this chapter (Section 18-2)
The chemical behavior of sulfides contrasts with that of ethers in some important ways. Because sulfur's valence electrons involve 3P orbitals they are further away and less tightly held than the valence electrons on oxygen which involve 2P orbitals. This makes the nucleophilicity of sulfur atoms much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. For instance, sulfides, unlike ethers, easily react with primary alkyl halides through an SN2 mechanism to give ternary sulfonium ions (R3S+) in the same manner that 3º-amines can be alkylated to form quaternary ammonium salts. Although equivalent oxonium salts of ethers are known, they are only prepared under extreme conditions, and are exceptionally reactive.
In biological systems the sulfonium ion, S-adenosylmethionine (SAM), is formed by a SN2 reaction between the nucleophilic sulfur atom of the amino acid methionine and the electrophilic methene (CH2) carbon of adenosine triphosphate (ATP). This reaction is unusually in that the triphosphate ion is removed from ATP as a leaving group. In most cases biological nucleophiles attack an electrophilic phosphorus on ATP causing a diphosphate ion to be removed as the leaving group.
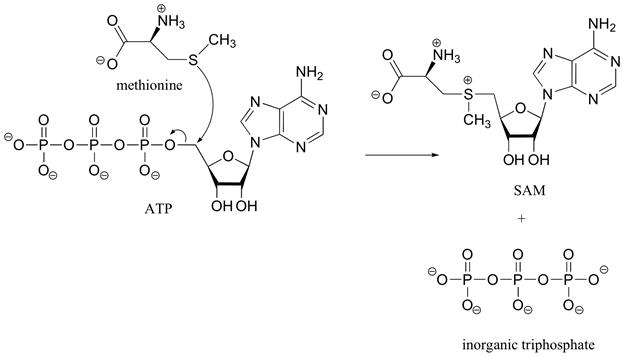
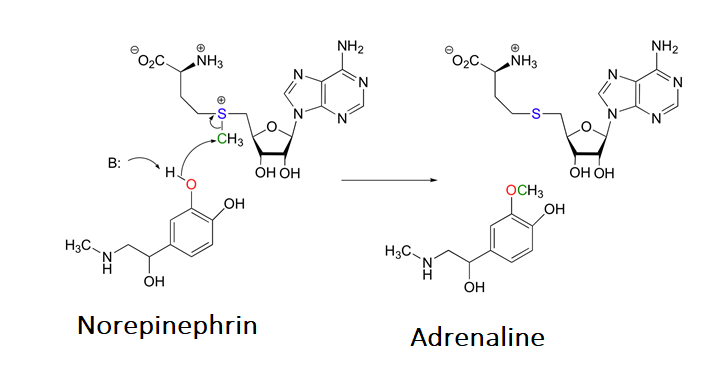
The sulfonium ion in SAM is an effective methylating agent. Through an SN2 reaction, biological nucleophiles can attack the electrophilic methyl group bonded to the positively charged sulfonium ion and cause the removal of a neutral sulfide as a leaving group. An example of this reaction is the catechol-O-methyltransferase catalyzed O-methylation of norepinephrin by SAM to produce adrenaline.
Sulfides can be easily oxidized. Reacting a sulfide with hydrogen peroxide, H2O2, as room temperature produces a sulfoxide (R2SO). The oxidation can be continued by reaction with a peroxyacid, such as peracetic acid (CH3CO3H), to produce the sulfone (R2SO2).
A common example of a sulfoxide is the solvent dimethyl sulfoxide (DMSO). DMSO is considered a polar aprotic solvent.
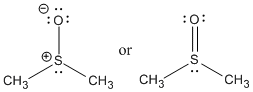
DMSO is a very polar, aprotic solvent
Give IUPAC names the following compounds:
a)
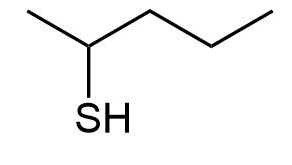
b)
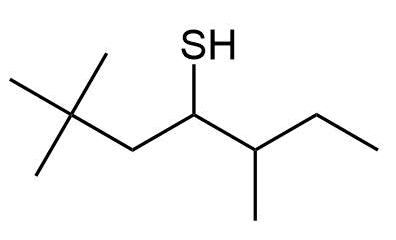
c)
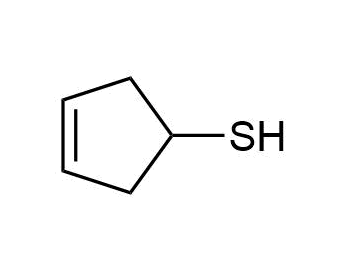
d)
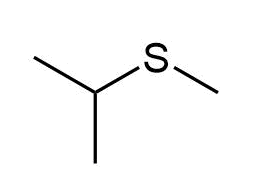
e)
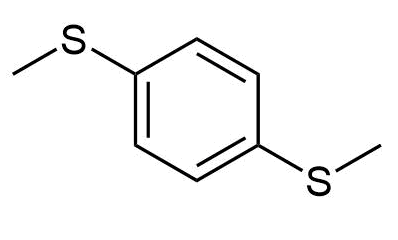
f)
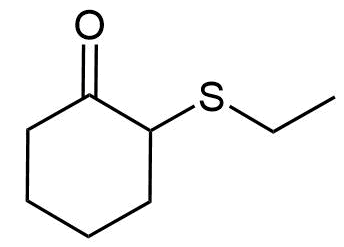
- Answer
-
a) 2-Pentanethiol
b) 2,2,5-Trimethyl-4-heptanethiol
c) 3-Cyclopentene-1-thiol
d) Methyl isopropyl sulfide
e) p-Di(methylthio)benzene
f) 2-(Ethylthio)cyclohexanone
The molecule 3-methyl-1-butanethiol has been shown to be one of the components of skunk spray. How would make the following conversions?
a)
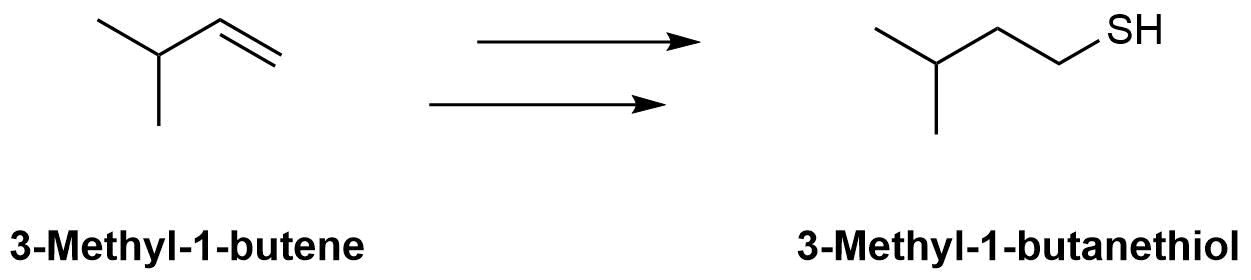
b)
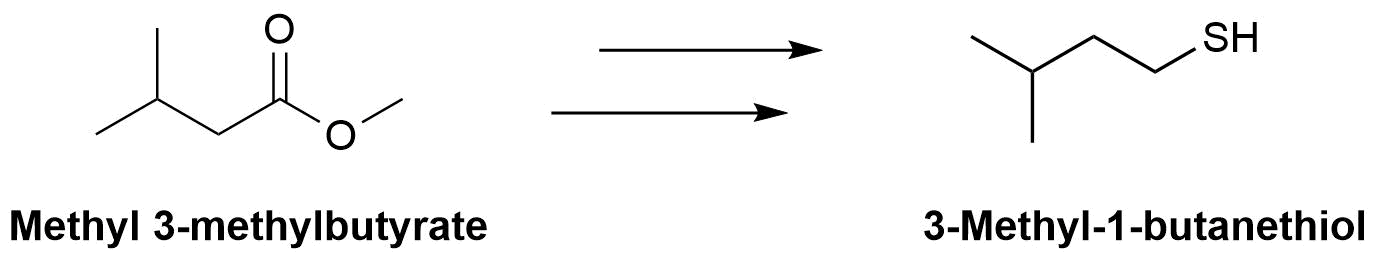
- Answers
-
a) 1) HBr & Peroxides, 2) (NH2)2C=S, 3) NaOH & H2O
b) 1) LiAlH4, 2) PBr3, 3) (NH2)2C=S, 4) NaOH & H2O

