4.1: Biosynthesis of Monoterpenes - Loganin
- Page ID
- 285449
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Terpenes have a large variety of carbon skeletons characterized by branched chains, and often complex multicyclic ring systems. They are oligomers of the biological isoprene unit, Δ3-isopentenol, which is a relatively reduced hydrocarbon comprised of five carbons. It is produced in nature from three molecules of a relatively oxidized two carbon starting material, acetic acid in the form of acetyl CoA. A likely candidate for the byproduct containing the carbon atom lost from three molecules of acetic acid during the biosynthesis of Δ3-isopentenol is carbon dioxide. Polar analysis suggests a more highly oxidized precursor, mevalonic acid, that could be decarboxylated by polar fragmentation of a \(\ce{CO2}\) electrofuge and a hydroxide nucleofuge. Such a fragmentation can benefit from the thermodynamic advantage of generating a C=O bond and produces easily disposable highly oxidized byproducts, \(\ce{CO2}\) and water. Further retrosynthetic analysis of mevalonic acid suggests polar disconnection to two acetic acid carbanion synthons which would condense with an acetyl electrophile.

A very large variety of lipids are derived in nature from the oligomerization of Δ3-isopentenyl pyrophosphate (5). This five carbon biosynthetic building block is produced by condensation of three molecules of acetyl CoA. Acetoacetyl CoA (1), produced by Claisen condensation of two molecules of acetyl CoA, reacts at the ketone carbonyl with a second equivalent of acetyl CoA as nucleophile. This condensation is enantioselective. The asymmetry of the enzyme, hydroxymethylglutaryl CoA synthetase, directs the attack of the acetyl CoA nucleophile to one side of the prochiral acetoacetyl CoA electrophile. The product is symmetrical. Nevertheless, the condensation is accompanied by the enantioselective hydrolysis of the CoA-SH ester derived from the acetyl group. The monothioester 2 is then reduced by hydride attack at the more electrophilic thioester carbonyl to give L-mevalonic acid (3). Phosphorylation of 3 leads, via a 5-monophosphate and 5-pyrophosphate, to an unstable intermediate phosphorylated at the C- 3 hydroxyl. This tertiary phosphate readily undergoes decarboxylative elimination to give Δ3-isopentenyl pyrophosphate that readily isomerizes to Δ2-isopentylpyrophosphate.

A head to tail dimer, geranyl pyrophosphate (E-7), is formed by addition of the allylic electrophile 6 to the terminal olefin 5 accompanied by proton loss. The resulting ten carbon allylic pyrophosphate E-7 readily alkylates a second molecule of 5 to give a trimer, the fifteen carbon allylic pyrophosphate farnesyl pyrophosphate (E-8). The monoterpenes are C10 compounds biogenetically derived from geranyl pyrophosphate (E-7), its Z-isomer neryl pyrophosphate (Z-7), or from a cyclopropyl dimer, chrysanthemyl pyrophosphate (9), that is formed directly from two molecules of Δ2-isopentenyl pyrophosphate. Isoprene units are often discernable embeded in the skeletons of terpenes. However, some terpenes, e.g. derivatives of the santolinyl cation, are not composed entirely of intact isoprene units owing to rearrangements during their biosynthesis (vide infra). These are called irregular terpenes.

Intramolecular nucleophilic attack by a C=C π-bond on the electrophilic pyrophosphate generates various isomeric carbocationic intermediates such as menthane, pinane, carane, camphane, or thujane carbenium ions.

The monoterpene loganin is the biosynthetic precursor of secologanin, a natural product whose terpenoid origin is unobvious. Secologanin, whose isoprene units are not intact, is derived biosynthetically by a polar cleavage of the cyclopentane ring of loganin exploiting the polar activation afforded by the cyclo pentane hydroxyl substituent.

Polar analysis of loganin shows that the hydroxyl substituent is not essential for facilitating generation of the cyclopentane ring by a polar C-C bond formation since adequate functionality is located in the proximity of the key bond. In the biosynthesis of loganin, this hydroxyl group is introduced at the end of the synthesis by a remote oxidation. The dihydropyran is simply a derivative of a 1,5-dialdehyde whose structure is simplified by polar disconnection of a ring C-C bond located between two consonant functional groups. This dislocation represents a retro Michael addition. The requisite nucleophile could be generated by deprotonation of a saturated precursor. An alternative precursor for this nucleophile, the one involved in the biosynthesis, is an unsaturated aldehyde. Thus, conjugate addition of hydride to an a,b-unsaturated aldehyde provides the nucleophile which will be Michael alkylated. The highly oxidized cyclization substrate is derived from geraniol by multiple allylic oxidations, and geraniol is a dimer of two isopentenol precursors, Δ2 and Δ3-isopentenol.
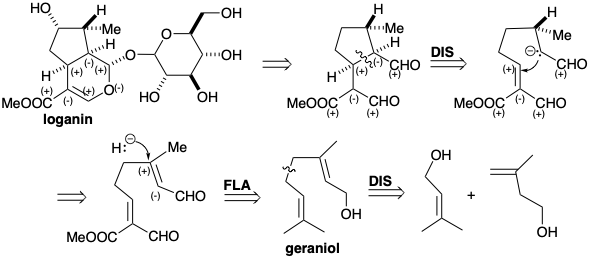
Loganin (13), the glucoside (a mixed acetal of glucose and an alcohol) of a monoterpene, is a key intermediate, which affords secologanin (14), the immediate precursor of the non-tryptamine portion of the corynanthe, aspidosperma, iboga, ipecacuanha and cinchona groups of indole alkoloids (see chapter 7). Loganin is produced from geraniol (10), which is first oxidized to a trialdehyde (11). Reductive cyclization of 11 to 12 is followed by further oxidations. Hydroxy loganin (14) gives secologanin (15) by a retro-Prins cleavage. The origin of secologanin from isoprenoid precursors is not immediately obvious from a cursory examination of its structure.



