1.3: Additions- Electrophilic and Nucleophilic
- Page ID
- 81769
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Addition of Water
Last time we listed three reasons to expect that the carbonyl (C=O) group would be a functional group, a reactive part of the molecule. All of these reasons were connected with the way electrons are distributed in the group. This makes sense because reactions involve making and breaking bonds. Bonds are electrons, so making and breaking bonds will change the location of electrons. Functional groups are the places where changing the location of electrons can happen fairly easily, which means that the distribution of electrons in a functional group is a key to its reactivity.
We need a specific example to make these ideas useful. We'll begin with the addition of water to a carbonyl group, specifically the aldehyde carbonyl group in acetaldehyde. The overall reaction (from reactants to products) is:
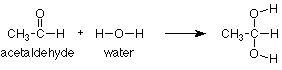
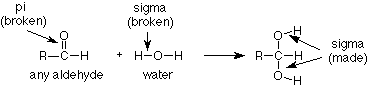
This type of reaction is known as an addition reaction. The name fits, because the product is the sum (or adduct) of the reactants. Addition reactions occur typically with functional groups which include pi bonds. Functional groups which include pi bonds are called unsaturated functional groups because some of the atoms in them have fewer than the maximum number of sigma bonds. For contrast, those which have no pi bonds do have the maximum number (four for carbon) of sigma bonds and are called saturated. (Take another look at the table of functional groups inside the front cover of Brown and note which ones are unsaturated.)
As we study more addition reactions of unsaturated functional groups during the semester, we'll notice that the pi bond in the reactant is typically broken. In the product we find that there are now two groups or atoms attached where the pi bond had been. In our instance, the pi bond between carbon and oxygen has disappeared and the hydrogen from water has added to the oxygen while the OH group from water has added to the carbon.
We can also notice that nothing happened to the CH3 group. It is saturated so it lacks a pi bond and cannot undergo an addition reaction. It is unreactive and is simply carried along from the reactant to the product.
Polarity Matching
Let's probe a little deeper into this reaction. Does it make sense that the OH group from water attaches to the carbon of the carbonyl? Does is make sense that the H from water attaches to the oxygen of the carbonyl? Let's look at the polarity of these materials.
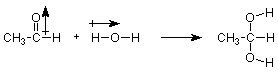
We notice that the positive (carbon) end of the carbonyl dipole becomes attached to the negative (oxygen) end of the OH dipole. Put another way, the electron-rich oxygen of water attacks the electron-poor carbon of the carbonyl group. Much of what we will learn in organic chemistry can be related to this idea, and we will develop it in more detail later in this lecture.
We noticed earlier that the CH3 group was not involved in any bond breaking or bond making. In this respect, it is very much like the "spectator ion" which did nothing in an inorganic reaction. These groups are caled "R-groups." Since these groups do not change in a reaction, we should focus our attention on the groups which do, the functional groups. For example, let's predict what the product of the following reaction would be:
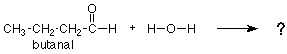
We might remember that the carbonyl group of acetaldehyde (above) adds water, the similar carbonyl group of butanal (below) should do so as well. We would look again at the acetaldehyde reaction, notice where the OH and H go, and put the OH and H in the same positions on the carbonyl group of butanal. We would get the following answer:
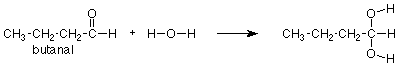
General Reaction
Notice again that the CH3CH2CH2 group didn't change and is merely copied from the reactant to the product. It helps immensely to think of this reaction as a reaction of the carbonyl group, not of the whole compound. In that way we can focus our attention on the part of the molecule which reacts and regard the rest as carried along for the ride. To express this in symbols, we can write the following equation which says that addition of water is a general reaction of the carbonyl group which occurs to any aldehyde.
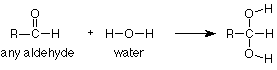
"R" is a "stand-in" for any group which might be attached to an aldehyde carbonyl. If a specific case tells us that R = CH3, then the complete equation is the first one we looked at (acetaldehyde). If a question tells us that R = CH3CH2CH2, as the butanal question did above, then we can use our general"R" reaction (directly above) and just replace "R" with CH3CH2CH2 whereever we see "R." This means that instead of learning every reaction of every compound, we only need to learn the reactions of the functional groups and how to apply them to specific cases as needed.
Mechanism
Now that we know something about how to use general ("R") reactions to tackle specific cases, let's turn to another question. How does this reaction take place? What sequence of events results in the breaking of the C-O pi bond and the O-H bond in water and the making of a new C-O sigma bond and a new O-H sigma bond?
Some experimental observations will help. We find that the addition of water to an aldehyde is rather slow if the solution is neutral (pH = 7, neither acidic nor basic), and that it is much more rapid if acid or base is added. In fact, the more acid or base is added, the faster the reaction goes. The acid or base is not used up, so what we are seeing is that the acid or base is acting as a catalyst.
Let's look at the acid catalyized case first and ask how an H+ might play a role in this reaction. Where would an H+ attack the carbonyl group? From our analysis of the C=O structure, we'd expect the electron-poor H+ to attack electrons on the electron rich oxygen of the C=O group. Since we need to break the pi bond, let's have the electrons of the pi bond move to make a new bond between O and H.
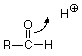
We symbolize that shift of electrons from between the carbonyl carbon and the carbonyl oxygen by drawing a curved arrow leading from the pi bond location to the new bond location between the carbonyl oxygen and the H+. That arrow can be interpreted to mean that the carbon loses one electron (one half of one pair) and the hydrogen gains one electron. The oxygen neither gains nor loses electrons since the electron pair stays connected to it. The outcome of this transaction is:
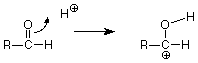
Keeping score, we have broken the carbon-oxygen pi bond, and we have made the new carbon-hydrogen sigma bond. We still have to make another bond, and the positively charged carbon atom with only three bonds looks like a reactive place. We need to make a bond between that carbon and the oxygen of water, but where do we get the electron pair needed to make that bond? The carbon is a poor candidate, so let's look at the oxygen. When we're looking for electrons to make a bond, we should consider unshared pairs. These are typically found on atoms like oxygen and nitrogen, although we don't usually draw them in unless we need them. Does such a pair exist on the oxygen of water? If we do our electron calculations carefully, the answer is yes, and that pair attacks the positively charged carbon atom. (Water is acting as a Lewis base, an electron pair donor, and the carbon is acting as a Lewis acid, and electron pair acceptor. The carbon has only six electrons in its sigma bonds and has a vacancy for an electron pair. Check the formal charge to verify this.)
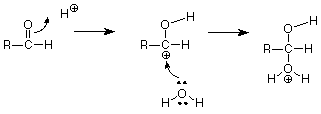
This makes the final carbon-oxygen bond. What remains (if we compare this structure with our product) is to break the final carbon hydrogen bond. Doing so, with that bond's electrons becoming an unshared pair on oxygen, gives us an H+ to replace the one which started the reaction so that the catalytic H+ is not used up. This sequence of steps by which the breaking and making of the requisite bonds is accomplished is called a mechanism.

If we summarize, another way to describe this is to say that the Lewis acid H+ attacks the electron pair of the carbon-oxygen pi bond. The positively charged carbon (carbocation) which results is also a Lewis acid and is attacked by the unshared pair of the oxygen of water, acting as a Lewis base. Finally the Lewis acid H+ is regenerated by cleavage of the oxygen-hydrogen bond in much the same way as H3O+ serves as a source of H+
Electrophile-Nucleophile
The terms Lewis acid and Lewis base are useful, but when we are talking about making and breaking bonds to carbons, we find that two other terms are more general. We use the term electrophile to designate atoms or groups which form bonds by using electron pairs from another atom. The positively charged carbon above is an example. It is attacked by the oxygen of water, using the oxygen's unshared pair. We use the term nucleophile to designate the atom or group which donates the electrons to make such a new bond to carbon. In our example, the oxygen atom is serving as a nucleophile. Another way to say this is that nucleophiles make bonds using their own electron pairs. Electrophiles make bonds using the electron pairs of nucleophiles. We can identify those roles in our mechanism as follows:
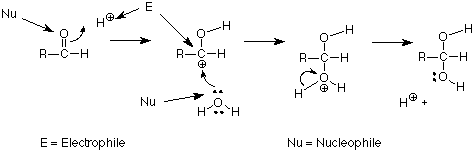
A summary of the reactivity of the carbonyl group is that electrophiles attack the oxygen; nucleophiles attack the carbon. We will find this to be a very useful way to organize what we learn about many other reactions of carbonyl groups.
Now, use these principles to work out a mechanism for the base catalyzed addition of water to a carbonyl group. Some suggestions: categorize the OH-- as a nucleophile or electrophile. Remember to break the pi bond to avoid having five bonds to carbon. Keep track of changes in the charge on particular atoms.


