8.7: Cycles of Matter
- Page ID
- 285332
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The physical connections among the environmental spheres are largely through cycles of matter. Involving physical processes, chemical reactions, and biochemical processes, they are called biogeochemical cycles. They are commonly named for the major element involved in each, usually an essential organism nutrient including carbon, nitrogen, oxygen, sulfur, and phosphorus. A particularly important cycle is the hydrologic cycle of water shown in Figure 8.1. In Figure 8.5 aspects of the rock cycle are shown in which molten rock solidifies, undergoes weathering, maybe carried by water and deposited as sedimentary rock, is converted to metamorphic rock by heat and pressure, and is eventually buried at great depths and melted to produce molten rock again. Anthrospheric processes are also very much involved in the important cycles of matter such as the input of chemically fixed nitrogen synthesized from atmospheric N2 in the nitrogen cycle.
The two major parts of biogeochemical cycles are the reservoirs in which matter is contained for some time and the conduits through which matter and energy are moved. Oceans, the atmosphere, parts of Earth’s crust, and organisms are reservoirs of matter. The atmosphere acts as a conduit to carry water vapor in the hydrologic cycle and streams carry sedimentary matter in the rock cycle. Endogenic cycles, of which the phosphorus cycle is an example, are those that occur below or directly on the surface of the geosphere without a significant atmospheric component; most biogeochemical cycles are exogenic cycles in which the atmosphere serves as a conduit and often as a reservoir.
Figure 8.8 illustrates one of the key biogeochemical cycles, the carbon cycle. An important reservoir of carbon is in the atmosphere as carbon dioxide. Photosynthetic processes by plants extract significant amounts of carbon from the atmosphere and fix it as biological carbon in the biosphere. In turn, animals and other organisms in the biosphere release carbon dioxide back to the atmosphere through the respiration processes by which they utilize oxygen and food for energy production. More carbon dioxide is released to the atmosphere by the combustion of biological materials such as wood and fossil fuels including coal and petroleum. Carbon dioxide from the atmosphere dissolves in water to produce dissolved carbon dioxide and inorganic carbonates. Solid carbonates, particularly limestone, dissolve in bodies of water to also produce dissolved inorganic carbon. Respiration of organic matter by organisms in water and sediments also
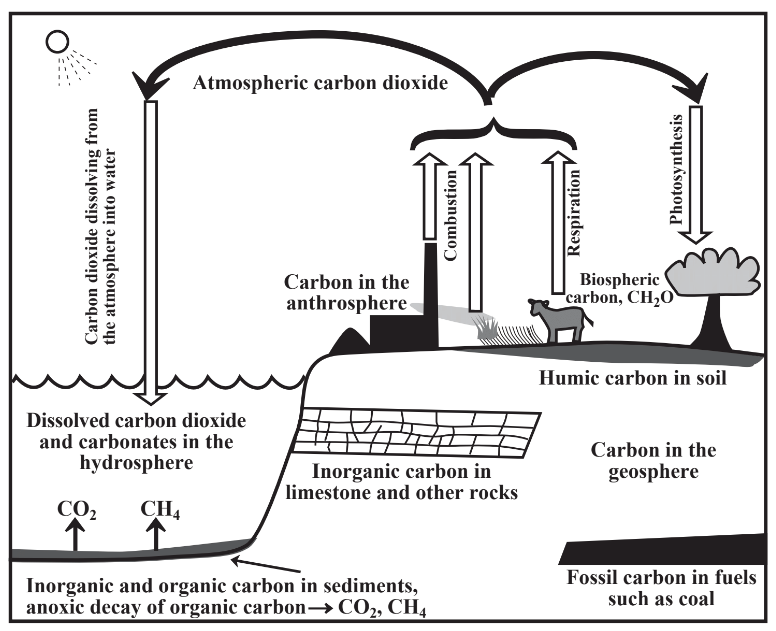
produces dissolved inorganic carbon species in water. Large amounts of carbon are held in the geosphere. The major forms of this carbon are fossil carbon of ancient plant origin held by fossil fuels, such as coal and petroleum, and inorganic carbon in carbonate rocks, especially limestone. Organic carbon from partially degraded plant material is present as humic material in soil, a source that is added to by organisms breaking down plant biomass from the biosphere.
The carbon cycle is extraordinarily important in maintaining sustainability because a major part of it is the fixation of carbon from highly dilute atmospheric carbon dioxide into biomass by photosynthesis carried out by green plants. Biomass is a source of food, chemical energy, and raw materials and the carbon cycle contains the main pathway by which solar energy is captured and converted to a form of energy that can be utilized by organisms and as fuel.
Other important cycles of matter are linked to the carbon cycle. The oxygen cycle describes movement of oxygen in various chemical forms through the five environmental spheres. At 21% elemental oxygen by volume, the atmosphere is a vast reservoir of this element. This oxygen becomes chemically bound as carbon dioxide by respiration processes of organisms and by combustion. The reservoir of atmospheric oxygen is added to by photosynthesis. Oxygen is a component of biomass in the biosphere and most rocks in Earth’s crust are composed of oxygen-containing compounds. With its chemical formula of H2O, water in the hydrosphere is predominantly oxygen.
In addition to the carbon and oxygen cycles described above, three other important life-element cycles are those of nitrogen, sulfur, and phosphorus:
•Nitrogen cycle: Biochemically bound nitrogen is essential for life molecules including proteins and nucleic acids. Although the atmosphere is about 80% by volume elemental N2, this molecule is so stable that it is difficult to split it apart so that N can combine with other elements. This process is performed in the anthrosphere by the synthesis of NH3, from N2 and H2 over a catalyst at high temperatures and very high pressures. Furthermore, air pollutant NO and NO2 form from the reaction of N2 and O2 under the extreme conditions in internal combustion engines. In contrast, some bacteria, including Rhizobium bacteria growing on the roots of legume plants, convert atmospheric nitrogen to nitrogen compounds under the very mild conditions just below the soil surface. Plants convert nitrogen in NH4+ and NO3- to biochemically bound N. As part of the nitrogen cycle, biochemically bound nitrogen is released as NH4+ by the biodegradation of organic compounds. The nitrogen cycle is completed by microorganisms that use NO3- as a substitute for O2 in energy-yielding metabolic processes and release molecular N2 gas to the atmosphere. Other than nitrogen fixation in the anthrosphere and formation of nitrogen oxides in the atmosphere from lightning discharges, most transitions in the nitrogen cycle are carried out by organisms, especially microorganisms.
•Sulfur cycle: The sulfur cycle includes both chemical and biochemical processes and involves all spheres of the environment. Chemically combined sulfur enters the atmosphere as pollutant H2S and SO2 gases, which are also emitted by natural sources including volcanoes. Large quantities of H2S are produced by anoxic microorganisms degrading organic sulfur compounds and using sulfate, SO42-, as an oxidizing agent and discharged to the atmosphere. Globally, a major flux of sulfur to the atmosphere is in the form of volatile dimethyl sulfide, (CH3S), produced by marine microorganisms. The major atmospheric pollutant sulfur compound is SO2 released in the combustion of sulfur-containing fuels, especially coal. In the atmosphere, gaseous sulfur compounds are oxidized to sulfate, largely in the forms of H2SO4 (pollutant acid rain) and corrosive ammonium salts (NH4HSO4) which settle from the atmosphere or are washed out with precipitation. The geosphere is a vast reservoir of sulfur minerals including sulfate salts (CaSO4), sulfide salts (FeS), and even elemental sulfur. Sulfur is a relatively minor constituent of biomolecules, occurring in two essential amino acids, but various sulfur compounds are processed by oxidation-reduction biochemical reactions of microorganisms.
•Phosphorus cycle: Unlike all the exogenous cycles with an atmospheric component discussed above, the phosphorus cycle is endogenous with no significant participation in the atmosphere. It is an essential life element and ingredient of DNA as well as ATP and ADP through which energy is transferred in organisms. Dissolved phosphate in the hydrosphere is an essential nutrient for aquatic organisms, although excessive phosphate may result in too much algal growth causing an unhealthy condition called eutrophication. Phosphorus is abundant in the geosphere, especially as the mineral hydroxyapatite, chemical formula Ca5OH(PO4)3. Significant deposits of phosphorus-rich material have be enformed from the feces of birds and bats (guano).


