7.9: Biochemistry of Toxic Substances and Toxicological Chemistry
- Page ID
- 285418
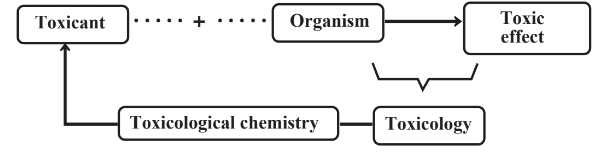
One of the more important aspects of biochemistry that is important in green chemistry is the way that organisms deal biochemically with toxic substances. There are two major aspects of this. One is the biochemical changes in toxic substances in an organism’s system including the detoxification of such substances and in some cases the conversion of nontoxic compounds to toxic compounds. The second is the biochemical mode of action of toxic substances through which they exert a toxic effect. Toxicological chemistry is the science that deals with the chemical nature and reactions of toxic substances, including their origins, uses, and chemical aspects of exposure, fates, and disposal.2 Toxicological chemistry addresses the relationships between the chemical properties and molecular structures of molecules and their toxicological effects. Figure 7.11 illustrates the definition of toxicological chemistry. This section discusses the biochemical aspects of toxicological chemistry. Toxicology, itself, and other details regarding toxicological chemistry are covered in Chapter 17.
Biochemistry of Toxicants and Protoxicants
In some cases a toxic substance that enters into the system of a living organism is unchanged until it reacts to cause a toxic effect. This is the case with carbon monoxide, CO, which enters the bloodstream through the lungs and binds with blood hemoglobin to prevent oxygen transfer to tissues. In other case toxicants or their metabolic precursors (protoxicants) react in ways that may make them more toxic or that detoxify them and facilitate their elimination from the organism. Xenobiotic compounds are those that are normally foreign to living organisms. Some of the more toxic substances, such as the toxin produced by Botulinus bacteria or the venom of the deadly Australian inland taipan viper, are among the most toxic substances known. “Toxicant” is used here as a term to refer to toxic substances and their precursors including both xenobiotic materials and those of natural organisms. “The body” is used to refer to the human body, but also applies to other organisms as well.
Of particular importance in the metabolism of toxicants is intermediary xenobiotic metabolism which results in the formation of somewhat transient species that are different from both those ingested and the ultimate product that is excreted. Intermediary metabolites may have significant toxicological effects. Toxicants and protoxicants in general are acted upon by enzymes that normally act upon an endogenous substrate, a material that is in the body naturally. For example, flavin-containing monooxygenase enzyme acts upon endogenous cysteamine to convert it to cystamine, but also functions to oxidize xenobiotic nitrogen and sulfur compounds.
Toxicants undergo biotransformation as a result of enzyme action, usually Phase I and Phase II reactions defined below. Some nonenzymatic transformations are also important including bonding of compounds with endogenous biochemical species without an enzyme catalyst, hydrolysis in body fluid media, or oxidation/reduction processes.
The likelihood of enzymatic metabolism in the body depends upon the physical and chemical properties of the species. Highly polar compounds, such as those that form ions readily, are less likely to enter the body system and generally are quickly excreted. Therefore, such compounds are unavailable, or only available for a short time, for enzymatic metabolism. Volatile compounds, such as dichloromethane or diethyl ether, are expelled so quickly from the lungs that enzymatic metabolism is less likely. This leaves as the most likely candidates for enzymatic metabolic reactions nonpolar lipophilic compounds, those that are relatively less soluble in aqueous biological fluids and more attracted to lipid species. Of these, the ones that are resistant to enzymatic attack (PCBs, for example) tend to bioaccumulate in lipid tissue.
Xenobiotic species may be metabolized in many body tissues and organs. The liver is of particular significance because materials entering systemic circulation from the gastrointestinal tract must first traverse the liver. As part of the body’s defense against the entry of xenobiotic species, the most prominent sites of xenobiotic metabolism are those associated with entry into the body, such as the skin and lungs. The gut wall through which xenobiotic species enter the body from the gastrointestinal tract is also a site of significant xenobiotic compound metabolism.
Phase I and Phase II Reactions
The metabolism of toxic substances may be divided into two phases. Phase I reactions normally consist of attachment of a functional group, usually accompanied by oxidation. For example, benzene, C6H6, (see Chapter 6, Section 6.2) is oxidized in the body by the action of the cytochrome P-450 enzyme system as shown in Figure 7.12.
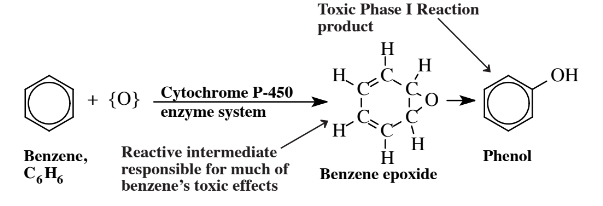
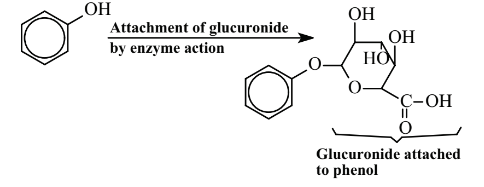
The Phase I oxidation product of benzene is phenol, a toxic substance. A reactive intermediate in the process is benzene epoxide, which interacts with biomolecules to cause toxic effects. The phenol Phase I oxidation product of benzene may undergo a second reaction, a Phase II reaction in which it is bound with a conjugating agent that is endogenous to (produced naturally by) the body, such as glucuronide (Figure 7.13).
Although Phase I and Phase II reactions generally act to make xenobiotic substances more water soluble, more readily eliminated from the body, and less toxic, in some cases, the opposite occurs and metabolic processes make substances more toxic. Most known human carcinogens(cancer-causing agents) are actually produced by biochemical processes in the body from non-carcinogenic precursor substances.
Biochemical and Toxic Effects of Toxicants
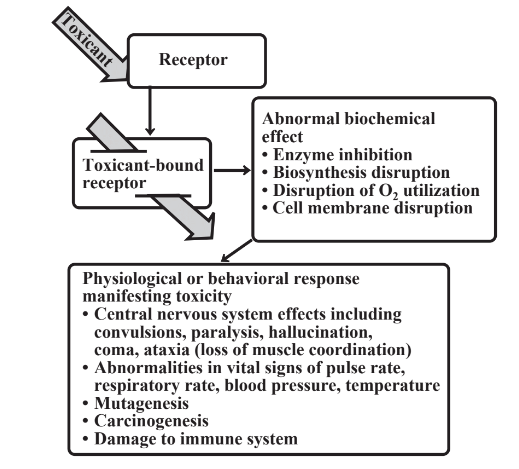
Toxic substances, which, as noted above, are often produced by metabolic processes from nontoxic precursors, produce a toxic response by acting upon a receptor in the body. Typically, a receptor is an enzyme that is essential for some function in the body. As a consequence of the binding of the receptor to the toxicant there is a biochemical effect. A common example of a biochemical effect occurs when a toxicant binds to an enzyme such that the bound enzyme may be inhibited from carrying out its normal function. As a result of a biochemical effect, there is a response, such as a behavioral or physiological response, which constitutes the actual observed toxic effect. Acetylcholinesterase enzyme inhibited by binding to nerve gas Sarin may fail to stop nerve impulses in breathing processes, leading to asphyxiation. The phenomena just described occur in the dynamic phase of toxicant action as summarized in Figure 7.14.
Toxicological Chemistry and the Endocrine System The endocrine glands and the hormones they produce (see Section 7.5 and Figure 7.6) are important in the consideration of toxicological chemistry, green chemistry and sustainability. Of particular importance are substances that organisms get exposed to through their environment, food, and drinking water that have the potential to disrupt the crucial endocrine gland activities that regulate the metabolism and reproductive functions of organisms. Hormonally active agents exhibit hormone-like activity that may be detrimental. Most commonly these are estrogenic substances that act like the female sex hormone estrogen. Some of these survive water treatment processes. Discharged to receiving waters they affect aquatic organisms and potentially can get into water that humans drink. Among such substances are estrogen, an endogenous sex hormone; 17a-ethinylestradiol, an ingredient of oral contraceptives; and chemicals from industrial and consumer sources that mimic estrogen. Estrogenic substances from artificial sources are called xenoestrogens and include antioxidants, bisphenol A, dioxins, PCBs, phytoestrogens (from plants), some pesticides (chlordecone, dieldrin, DDT and its metabolites, methoxychlor, toxaphene), preservatives, and phthalic acid esters (butylbenzyl phthalate).
The practice of green chemistry minimizes the production and use of xenoestrogens and attempts to prevent their introduction into the environment. A particular need exists for the development of alternatives to xenoestrogenic bisphenol A and phthalate plasticizers. These substances improve the properties of plastics and as molecules much smaller than those in the plastic polymer tend to get into the environment and food chain. Modification of the plastic polymer formulation to give desired properties without the need for plasticizer additives would be especially desirable.


