5.1: Describing What Happens with Chemical Equations
- Page ID
- 285297
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)How far would you have to go to find a diverse chemical factory carrying out hundreds of complex chemical processes? Not far, because your own body is just such a remarkably sophisticated factory that could not be duplicated by the efforts of thousands of chemists and chemical engineers and the expenditure of billions of dollars. As an example of a process that our bodies carry out consider the utilization of glucose sugar, chemical formula C6H12O6, which is present in our blood and generates energy that the human body uses by the following metabolic biochemical reaction:
\[C_{6}H_{12}O_{6} + 6O_{2} \rightarrow 6CO_{2} + 6H_{2}O\]
This is a chemical equation that represents a chemical reaction, something that actually occurs with chemicals. It states that glucose reacts with molecular oxygen to produce carbon dioxide and water. The chemical reaction also produces energy and that is why the body carries it out to obtain the energy needed to move, work, and grow. The production of energy is sometimes denoted in the equation by adding “+ energy” to the right side.
Just as a chemical formula contains a lot of information about a chemical compound, a chemical equation contains much information about a chemical process. A chemical equation is divided into two parts by the arrow, which is read “yields.” On the left of the arrow are the reactants and on the right are the products. A key aspect of a correctly written chemical equation is that it is balanced, with the same number of atoms of each element on the left as on the right. Consider the chemical equation above. The single molecule of C6H12O6 contains 6 C atoms, 12 H atoms, and 6 O atoms. The 6 O2 molecules contain 12 O atoms, giving a total of 18 O atoms among the reactants. Adding up all the atoms on the left gives 6 C atoms, 12 H atoms, and 18 O atoms among the reactants. On the right, the products contain 6 C atoms in the 6 CO2 molecules, 12H atoms in the 6 H2O molecules, and 12 O atoms in the 6 CO2 molecules, as well as 6 O atoms in the 6 H2O molecules, a total of 18 O atoms. So there are 6 C atoms, 12 H atoms, and 18 O atoms among the products, the same as in the reactants. Therefore, the equation is balanced.
An important exercise is the process of balancing a chemical equation. This consists of putting the correct numbers before each of the reactants and products so that equal numbers of each kind of atom are on both the left and right sides of the equation. The procedure for balancing a chemical equation is addressed in section 5.2.
Learning chemistry is largely an exercise in learning chemical language. In the chemical language the symbols of the elements are the alphabet. The formulas of the compounds are the words. And chemical equations are the sentences that tell what actually happens.
It is often important to know the physical states of reactants and products in chemical reactions. Suppose, for example, that a geologist tested a sample of rock to see if it were limestone by adding some liquid hydrochloric acid to the rock and observing the CO2 gas coming off. The equation for the chemical reaction that occurred is
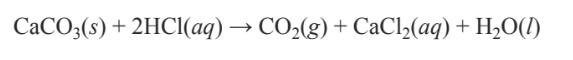
\[\ce{CaCO3(s) + 2HCl(aq) \rightarrow CO2(g) + CaCl2(aq) + H2O(l)}\]
Here abbreviations in parentheses are used to represent the physical state of each reaction participant — (s) for solid, (aq) for a substance in solution, (g) for gas, and (l) for liquid. The equation above states that solid calcium carbonate reacts with an aqueous solution of hydrochloric acid to produce carbon dioxide gas, a solution of calcium chloride, and liquid water.
Chemical reactions often are reversible, that is, they may go either forward or backward. A reversible reaction is shown with a doublearrow,←→. Asan example, consider the reaction of dissolved ammonia, NH3, with water to produce ammonium ion, NH4+, and hydroxide ion, OH-.
\[\ce{NH3(aq) + H2O(l) \rightleftharpoons NH4^{+} (aq) + OH^{-} (aq)}\]
Actually, only a small fraction of NH3 molecules undergo this reaction at any given time, and those that are converted to NH4+ are rapidly converted back to NH3. The double arrow in the chemical equation shows that both the forward and reverse processes occur. Another symbol that is sometimes used in chemical equations is ∆. This symbol denotes that heat is applied to make the chemical reaction occur at a more rapid pace. It is normally placed over the arrow in the chemical reaction. Chemical equations are used to calculate the quantities of chemicals involved in a chemical reaction, either as reactants or as products. This is an important area of chemistry that is addressed by the topic of stoichiometry discussed later in this chapter in Section 5.8.


