Introduction to GC
- Page ID
- 60979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Harold McNair, Virginia Tech, USA
Abstract: Chromatography is an excellent and extremely powerful separation technique. It is fast, simple, sensitive and usually requires little sample.
Level: Basic
Chromatography is carried out to separate an often complex sample mixture into its individual components and to obtain information in terms of:
- Qualitative analysis:
Which components are present in the sample? The identification of individual sample components can be assessed from the chromatogram. A parameter that provides information for the identification of a sample component is the retention time. - Quantitative analysis:
How much of each compound is present? Quantitative analysis involves measuring the amount - the concentration - of sample components. Concentrations can be determined from the peak area or the peak height in the chromatogram.
Chromatography is widely used in all kinds of applications area's, such as medical, environmental, food analysis, pharmaceutical, toxicology, quality testing and many more. Chromatography is becoming more and more refined and improved every day.
Video presentations called 'Introduction to GC' can be found by clicking this link.
Separation process in GC
Chromatography is a dynamic separation process based on differences in the rates of migration of different compounds through a column containing an immobilised phase. Migration is induced by a continuous flow of the mobile phase through the column.
In general chromatography is based on the selective distribution of the different components between two phases. One of the phases, the stationary phase, is held immobilised inside the column while the other phase, the mobile phase travels through the column, flowing through the stationary phase. Compounds that exhibit a higher affinity for the stationary phase will travel more slowly than compounds exhibiting a lower affinity. The different sample components travel at different speed through the column and are eluted from the column at different times. The column should be sufficiently long to obtain the desired degree of separation.
Role of separation
Separation techniques play an important role in modern analytical chemistry. Most chemical analyses involve the separation of complex samples. These mixtures contain at least one component in which we can be interested for different reasons e.g.:
- Assessment of a product
- Continuous monitoring of production process as part of quality control
- Monitoring and controlling industrial waste products as preventative quality control of the surface water
- Determination of amounts of pharmaceuticals and their metabolites in biological samples in clinical research of (new) drugs.
The components we wish to analyze (called analytes) are usually accompanied by other components that are generally present in much larger amounts. These other components are called the sample matrix. In general the nature and composition of this matrix is not of direct interest, but we have to take matrix effect into account. We speak of matrix effects if compounds present in the matrix affect sample pretreatment, separation or detection of the analytes.
Chromatography is often used to separate analytes from the matrix and to determine each analyte separately. Often the sample has to be treated - sample preparation - before we can start the chromatographic separation. Chromatography enables both qualitative (detection and identification) and quantitative analysis.
Chromatographic separation techniques
A number of separation techniques are available. The two most commonly used methods in modern chromatography are gas chromatography (GC) and high performance liquid chromatography (HPLC). HPLC has a number of different separation modes, of which the basics are described here. These techniques compliment each other with regard to their respective fields of application, even though there is some degree of overlap. One can say that GC is the more powerful technique with regard to separating very complex mixtures, providing that they are thermally stable, whereas HPLC is more versatile with respect to sample type and selectivity.
Common separation techniques are:
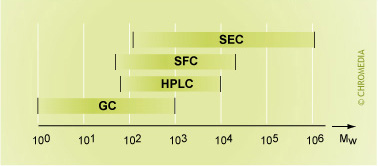
- Gas Chromatography (GC): GC is restricted to compounds with a molecular weight up to about 1000 in the vapour phase.
- Liquid Chromatography (LC): LC is applied for compounds with a molecular weight of 70 up to 1,000,000. Sample components must be soluble in the mobile phase.
- Size Exclusion Chromatography (SEC)
- Supercritical Fluid chromatography (SFC) uses CO2 - having both liquid and gas-like properties- as mobile phase.
Gas chromatographic separation is based on:
- Distribution (In gas-liquid chromatography, GLC)
- Adsorption (In gas-solid chromatography, GSC)
- Size exclusion (Molsieves)
Practical aspects
Gas chromatography can be used for the separation of volatile organic compounds. However, numerous solutes that should be determined in the bio-analytical or environmental field are not suitable for direct GC determination because of there limited stability at the elevated temperatures used during GC separations.
Scheme of Universal GC
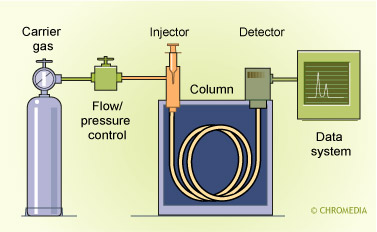
Derivatization with a suitable reagent can significantly improve the temperature stability of an analyte. A Derivatization step should be considered as an integral part of the total Sample Treatment/ Preparation procedure. During a derivatization reaction normally a polar function of the analyte is converted into a less polar functionality. Derivatization procedures, both for GC and LC, will be discussed extensively in one of the other Chapters of this Topic circle.
GC can be performed nowadays with the traditional techniques like the various modes of extraction, but also multi-dimensional approaches that can be performed either at-line or on-line are widely available. Especially the use of ‘pre-columns’ is gaining in popularity.
GCxGC scheme
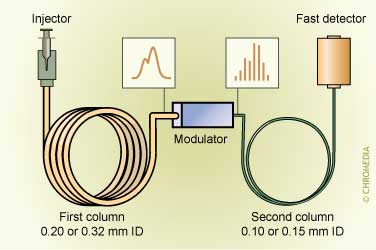
The popularity of GC in environmental analysis is due to fact that GC, in principle, is a more efficient a faster technique – compared with LC – and can be combined easily with a number of selective and sensitive detectors. Moreover, the analytes in environmental analysis are still relatively non-polar compared with the drugs that should be determined in biological samples.
Stationary phases
The key parameter in performing a good GC separation is choosing the optimum stationary phase and column and optimum flow rate of the carrier gas and optimum temperature or temperature program belonging to the chosen set of hardware and the physico-chemical properties of the analyte(s).The fact that the choice of the sorbent in more critical in GC as in LC is due to the fact that in GC no mobile phase is present, the carrier gas mainly act as a transport medium and there are hardly any selective reactions between the analytes and the carrier gas and the carrier gas and the stationary phase.
Rapid temperature programming in gas chromatography
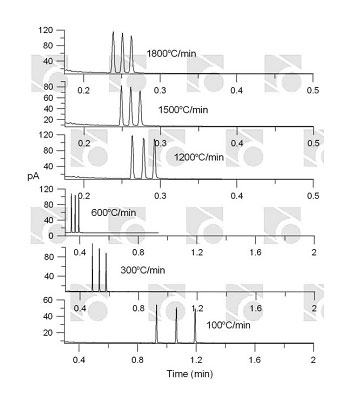
The following features are important in choosing the proper separation system:
- The boiling point range of the analytes and the properties of the resulting vapours phase of these analytes;
- The number of components that must be separated;
- The presence of polar and/or non-polar groups in the analytes and the presence or absence of functional groups;
- The combination of stationary phase and column dimensions as well as the film thickness of the stationary phase with respect to the required selectivity and resolution;
- The flow rate of the carrier gas as a parameter to speed up the total analysis, with a minimum loss in resolution of the most critical pair of compounds to be separated;
- The fact that either a temperature program should be used or that the analysis can be performed at a constant temperature.
Stationary phases in GC can be divided based on their polarity or selectivity with respect to one or more physico-chemical properties of the analyte molecules such as molecular mass or molecular shape, boiling point or the presence of certain functional groups (table herunder).
First of all the stationary phases available for packed columns will be discussed and later on, the nowadays far more popular, sorbents for capillary columns will be discussed.
The separation on the non-polar sorbents like SE-30, OV-1 and Apiezon L, a branched alkane polymer with a melting point of 430C, is primarily based on molecular size and shape. In general the elution order corresponds to the molecular mass of the compounds. In particular for neutral and weakly acidic compounds these phases are suitable.
|
Stationary phase |
Trade names |
Maximum |
Applications |
|---|---|---|---|
|
Polydimethyl siloxane |
OV-1, SE-30 |
350 |
General-purpose non-polar phase; hydrocarbons; polynuclear aroma-tics; drugs; steroids; PCBs |
|
Poly(phenylmethyldimethyl) siloxane (10% phenyl) |
OV-3, SE-52 |
350 |
Fatty acid methyl esters; alkaloids; drugs; halogenated compounds |
|
Poly(phenylmethyl) siloxane (50% phenyl) |
OV-17 |
250 |
Drugs; steroids; pesticides; glycols |
|
Poly(trifluoropropyldimethyl) siloxane |
OV-210 |
200 |
Chlorinated aromatics; nitroaroma-tics; alkyl-substituted benzenes |
|
Polyethylene glycol |
Carbowax 20M |
250 |
Free acids; alcohols; ethers; es-sential oils; glycols |
|
Poly(dicyanoallyldimethyl) siloxane |
OV-275 |
240 |
Polyunsaturated fatty acids; rosin acids; free acids; alcohols |
Source (D.A. Skoog, D.M. West, F.J. Holler, S.R. Crouch, Fundamentals of Analytical Chemistry, Thomson, Belmont, 2004).
The polyethyleneglycol (PEG) stationary phases are the most frequently used polar sorbents. PEG 400 is a suitable sorbent for the separation of alcohols, ethers, aldehydes and other compounds with low boiling points, while Carbowax 20M can be used for the separation of polar compounds with higher boiling points.
These phases are in particularly useful for the determination of strong basic compounds. Another group of polar stationary phases are the poly(cyanopro-pylphenyldimethyl) siloxanes. These moderately polar sorbents are used for the separation of compounds containing several hydroxyl groups (e.g. steroids).
In summary it can be stated by using only a limited number of sorbents, most separations can be performed in case temperature programming is applied:
Analytes possessing non-polar groups: OV-101, Apiezon L
Analytes with moderately polar groups: OV-1, OV-1701
Analytes with polar groups: Carbowax 20 M.
As mentioned before different type of columns are used for GC separations:
Cross-section of capillary columns
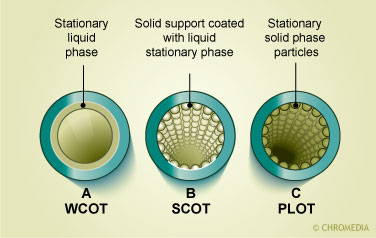
In the picture a packed column with an internal diameter of 2-4 mm and a tubular construction of either glass or stainless steel can be seen. The column is packed with particles which either act as the retarding stationary phase or are coated with a thin film of organic material which acts as the stationary phase. The other pictures depict open tubular columns. These columns have a narrow internal diameter between 0.17-0.53 mm. There are two major types of capillary columns: the porous layer open tubular (PLOT) columns in which the inner surface of the column has an embedded layer that contains the stationary phase and the wall coated open tubular (WCOT) in which a thin film of organic stationary phase is bonded directly onto the inner surface of the column. A comparison between packed and capillary columns is given in the table.
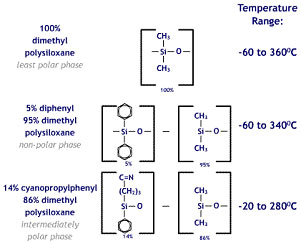
The chemical structure of some of the stationary phases described in this table is pictured here. The non-polar phases are more stable and chromatographically robust than the polar phases which tend to have a lower temperature tolerance and are more susceptible to oxidative damage if air is introduced into the column.
|
Stationary phase |
Equivalent packed column |
Structure of side chains of the poly-siloxane |
Polarity |
Applications |
|---|---|---|---|---|
|
X-1 |
OV-101, SE-30 |
100% methyl |
Non-polar |
Solvents, petroleum pro-ducts, volatile organic com-pounds, environmental con-taminants, amines, drugs |
|
X-5 |
SE-54 |
5% phenyl, 95% me-thyl |
Non-polar |
PAHs, perfume components, environmental contaminants, drugs, |
|
X-1701, X-10 |
OV-1701 |
14% Cyanopropyl, 86% methyl, 50% phenyl |
Moderately polar |
Pesticides, alcohols, phenols, esters, ketones |
|
X-17 |
OV-17 |
50% phenyl |
Moderately polar |
Drugs esters, ketones, plas-ticizers, organochlor com-pounds |
|
X-200, X-210 |
OV-210 |
50% trifluoropropyl, 50% methyl |
Polar |
Selective for compounds with free electron pairs, ste-roids, esters, ketones, drugs, alcohols, freons |
|
X-WAX |
Carbowax 20M |
polyethyleneglycol |
Strongly polar |
Alcohols, methylesters of fatty acids, solvents, fatty acids, amines |

