GC-MS: Performance Enhancing Drugs
- Page ID
- 287959
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Gas Chromatographic Separations of Steroids
Purpose: Introduce the students to the concepts related to gas and liquid chromatographic separations and elucidate the mechanisms through which different separations take place.
Learning Outcomes:
By the end of the assignment students will be able to:
- Differentiate between GC stationary phases and their selectivities.
- Identify the factors that influence retention of analytes.
- Recognize the relationship between chromatographic peak shape and various chromatographic influences.
- Identify the advantages and disadvantages of the derivatization of analytes for GC volatility.
The most common gas chromatography columns used today are long (e.g., 30 or 60 m) fused silica capillary tubes. The stationary phase typically consists of a polymeric liquid that is first coated onto and then chemically bonded onto the underlying silica surface of the capillary tube. The stationary phase is chosen for its interaction with the compounds to be separated and for its stability at the desired temperature for the separation. As a compound in the gas phase moves through the column encountering the bonded phase, it will spend time partitioned into the bonded phase due to physical interactions with that material. The magnitude of a compound’s interaction with the stationary phase can be expressed as a partition coefficient, compounds with sufficiently different partition coefficients can be separated by a chromatographic column. The smaller the partition coefficient, the less time the compound spends within the stationary phase and the faster it elutes from the column.
Once a stationary phase has been selected, it is important to be able to predict the elution order (the order in which compounds will emerge from the column) for the chemical compounds we wish to separate. In gas chromatography, one possibility is to consider the differences in the boiling points of compounds. The boiling point of a pure compound is dependent on the intermolecular interactions between the molecules. The stronger these interactions, the higher the boiling point. Boiling points of linear alkanes are primarily controlled by dispersion forces. The more atoms in the alkane, the greater the number of polarizable electrons, the greater the energy of interaction, consequently the higher the boiling point of the alkane. Table 1 gives the boiling points of a series of linear alkanes which provides an indication of how the size of a molecule can influence its volatility.
Table 1. Linear Alkane Data (http://webbook.nist.gov/chemistry/)
| Compounds | # C atoms | Mass | Boiling Point °C | Boiling Point K |
|---|---|---|---|---|
| Propane | 3 | 44.0956 | -42.0°C | 231.1 |
| Butane | 4 | 58.122 | -0.5 | 272.5 |
| Pentane | 5 | 72.148 | 36.0 | 309.2 |
| Hexane | 6 | 86.17 | 68.8 | 341.9 |
| Heptane | 7 | 100.2 | 98.4 | 371.5 |
| Octane | 8 | 114.22 | 125.6 | 398.7 |
| Nonane | 9 | 128.255 | 150.6 | 423.8 |
| Decane | 10 | 142.28 | 174.0 | 447.2 |
| Undecane | 11 | 156.3083 | 195 | 468 |
| n-dodecane | 12 | 170.3348 | 216 | 489 |
| Tridecane | 13 | 184.3614 | 234 | 507 |
| Tetradecane | 14 | 198.3880 | 250 | 523 |
| Pentadecane | 15 | 212.4146 | 267 | 540 |
Not all molecules can be volatilized directly, many will decompose at high temperatures, resulting in very little of the intact compound entering the gaseous state. In other cases, despite being volatilized, the analytes yield very poor signal responses in the GC detector. To overcome these difficulties, specialized reagents can be employed to derivatize the target analytes, rendering them more stable, more volatile, or more easily detected. The selection of derivatizing agent follows the intended objective of the derivatization procedure and target analyte; the most common classes of reactions are silylation, alkylation and acylation.
Steroid analysis is often necessary for the determination of doping by athletes. The anti-doping test is done by examining the steroids found in the urine of athletes, looking to identify if there are excess amounts of native steroids (i.e. testosterone) or the presence of synthetic steroids (e.g. nandrolone, stanozolol,…). To perform this analysis the collected steroids will need to be converted to gasses. This is not a trivial process as the steroids generally have too high a boiling point, or decompose above their melting point. The use of proper derivatization of the steroids will be crucial for their analysis. Once in the gas phase the compounds will need to be passed down a column capable of differentiating between the rather similar structures of the steroids, and in some cases, enantiomeric forms of the compounds. As such the choice of the column stationary phase will be crucial to obtaining resolved separations.
Gas Chromatography
- What would you expect to see as the output from a gas chromatographic (GC) separation?
- In figure 1 (below) a number of peaks have been labelled (C12, C13, C14 & C15) representing the n-alkanes in the sample. How might these specific peaks have been identified as the respective compounds, rather than the other peaks in the chromatogram?
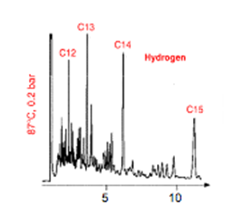
- From the table above, plot the boiling points of the alkanes versus their mass. Describe any trend(s) you observe in your graph.
- Figure 1 (above) contains a portion of chromatogram showing the elution of a sample of n-alkanes from a column held at a constant temperature of 87°C. Estimate the approximate time at which C16 will appear?
- Does a GC column need to be above the boiling point of the analytes in order for them to be moved down the column and separated?
- How would you expect the elution times of the peaks (C12-C15) to change if you increased the column temperature to 120°C?
- What would the chromatogram from figure 1 look like (with respect to the n-alkanes C12-C15) if the separation was started with a temperature of 87°C and held at that temperature for five minutes, then the temperature was rapidly increased to 120°C?
For the analysis of steroids by GC-MS the steroids must be derivatized prior to injection on the column. This is most commonly done with a silyating agent, such as N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA), which will react with active hydrogen (eg. -NH, -COOH, -SH & -OH hydrogens); the hydrogens on these groups are replaced with trimeythylsilane (-S(CH3)3) as depicted in the reaction below:
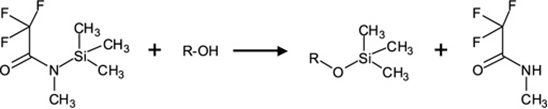
- What is the principle change, in terms of molecular interactions, in a molecule such as testosterone if a hydroxyl group reacts to add a trimethylsilane (TMS) group?
- What advantage(s) does this change yield to the separation of steroids such as testosterone by GC?
- Ethinylestradiol (see structure below) is a derivative of the hormone estradiol and similar in structure to many steroids. If ethinylestradiol was derivatized with MSTFA what product(s) would be formed? What would a GC separation of the derivatized product(s) look like?

- Can you envision any other ways that the derivatization of analytes could be advantageous?
- The separation of linear alkanes is accomplished on the basis of the differences in boiling points of the compounds. On what basis could compounds with very similar boiling points be separated by GC?
The table below lists a range of column types used in GC. Each column type provides unique selectivity for the separation of analytes, consequently some columns are better suited to certain classes of analytes than others.
Selected Examples of Stationary Phases for Gas-Liquid Chromatography
| stationary phase | polarity | trade name | temperature limit (oC) | representative applications |
|---|---|---|---|---|
| squalane | nonpolar | Squalane | 150 |
low-boiling aliphatics hydrocarbons |
| Apezion L | nonpolar | Apezion L | 300 |
amides, fatty acid methyl esters, terpenoids |
|
polydimethyl siloxane |
slightly polar | SE-30 | 300–350 |
alkaloids, amino acid derivatives, drugs, pesticides, phenols, steroids |
|
phenylmethyl polysiloxane (50% phenyl, 50% methyl) |
moderately polar | OV-17 | 375 |
alkaloids, drugs, pesticides, polyaromatic hydrocarbons, polychlorinated biphenyls |
|
trifluoropropylmethyl polysiloxane (50% trifluoropropyl, 50% methyl) |
moderately polar | OV-210 | 275 |
alkaloids, amino acid derivatives, drugs, halogenated compounds, ketones |
|
cyanopropylphenylmethyl polysiloxane (50% cyanopropyl, 50% phenylmethyl) |
polar | OV-225 | 275 |
nitriles, pesticides, steroids |
|
polyethylene glycol |
polar | Carbowax 20M | 225 |
aldehydes, esters, ethers, phenols |
- For each of the column types listed in the table above determine the structure of the monomer(s) that comprises the polymer stationary phase.
- Why is there a temperature limit for each type of column? What would happen to the chromatograms if the temperature limit of the column was exceeded?
- Three analytes (A, B & C) have very similar boiling points; however, they differ significantly in terms of polarity (A is the most polar, C is the least polar). Predict the elution order for the separation of A, B and C on each of the columns below:
- Squalane
- OV-17
- Carbowax 20M
- Two stationary phases in the Table (polydimethyl siloxane and cyanopropylphenylmethyl polysiloxane) are listed as being suitable for the separation of steroids. Provide a rationale for why these two phases are suitable for the separation of steroids.
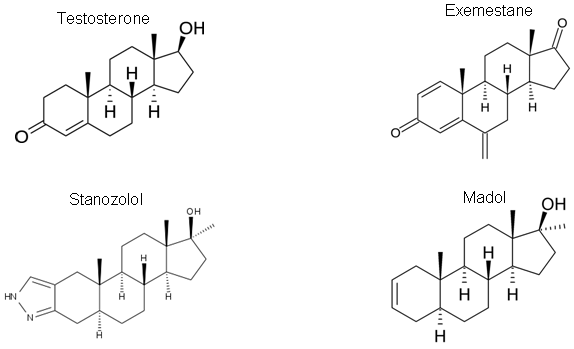
- Can you predict the elution order for the MSTFA derivatives of the steroids below on a polydimethyl siloxane column? What additional information might you need to be sure of your predictions?
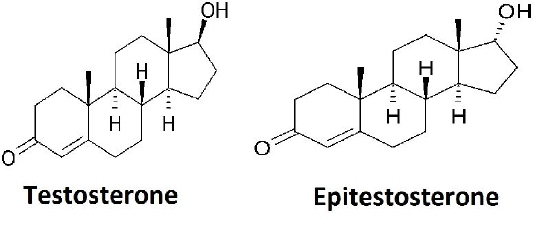
- In addition to testosterone, the body also produces an enantiomer of testosterone known as epitestosterone. As testosterone and epitestosterone are enantiomers, do you expect to be able to separate them by GC? Why or why not?
- Can you think of a way that you could separate enantiomers using GC?
- With GC the amount of analyte in a sample is deemed to be proportional to the peak area assigned to that compound. In order for there to be proper quantification of an analyte can its peak overlap with another peak? If so, how much overlap is acceptable?
- In the chromatogram below solutes 1 and 2 do not appear to be fully resolved. How would you go about quantifying each peak ?
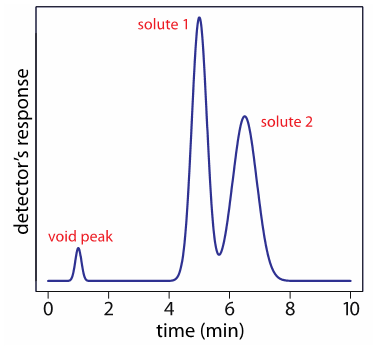
The extent of overlap, or separation, between two peaks in a chromatogram is described as the resolution (RS) between the peaks. This value is quantified on the basis of the peak width, and the elution time for the peak (see equation below). Based on the value obtained through the calculation peaks can be described as being baseline resolved (fully separated) or not.
\[R_S = \dfrac{2[(t_R)_B-(t_R)_A]}{W_A+W_B}\nonumber\]
- A pair of peaks on a chromatogram are said to be baseline resolved when the measured resolution between the peaks is greater than, or equal to, 1.5. Derive how a value of 1.5 is deemed to be baseline resolved (Hint: think of the width/shape of a Gaussian curve and its standard deviation.)
- Under ideal circumstances, the shape of the peak for an analyte from a GC separation will be Gaussian shaped. However, a number of factors can cause the peaks not to be Gaussian, typical variations are either fronted or tailed peaks. From the list tabulated below determine how each factor would alter the peak shape and explain why.
|
Contribution |
Peak Shape |
Reason |
|---|---|---|
|
Secondary retention mechanism. |
|
|
|
Too large a sample volume. |
|
|
|
Too high a sample concentration. |
|
|
|
Too low a column temperature. |
|
|
|
Loss of column stationary phase. |
|
|
|
Too low an injection port temperature. |
|
|
The elution of compounds from a GC column is a predictable process. For example, a given series of n-alkane compounds will always elute in the same order, from the shortest to the longest compound.
- How could you use the fact that analytes elute in predictable orders on similar stationary phases be useful in the identification of illegal steroids on new GC columns?
The fact that n-alkanes will elute in a predictable order has been used to generate a relative retention classification system, known as the Kovats retention index. This index is described by the equation below; wherein a value (Icpd) can be assigned to any analyte that elutes between a sequential pair of n-alkane compounds of chain length x and x+1 based on the adjusted retention time of the three components. Ix is the Kovats retention index for the shorter of the two n-alkanes which bracket the analyte compound; the Kovats retention index number of n-alkanes is the number of carbons in the chain multiplied by one hundred (e.g. hexane = 600, dodecane = 1200). Knowledge of the Kovats retention index for an analyte compound (Icpd) can be useful in comparing or predicting separations between different columns and instruments, so long as the separation conditions (stationary phase, temperature, column length...) remain the same.
\[I_\textrm{cpd}=100\times\dfrac{\log t^{'}_\textrm{r,cpd}-\log t^{'}_{\textrm{r},x}}{\log t^{'}_{\textrm{r},x+1}-\log t^{'}_{\textrm{r},x}}+I_x\nonumber\]
- The separation of testosterone and nandrolone are tested on a new GC column; under the separation conditions they are found to have adjusted retention times of 4.87 and 7.03 minutes respectively. A series of n-alkanes separated on the same column and conditions yields the following retention data (below). Determine the Kovats retention data for testosterone and nandrolone.
- If a steroid has a Kovats retention index of 823 when would you expect it to elute from the column whose data is tabulated below?
|
n-alkane |
Time |
|---|---|
|
C5H12 |
1.64 |
|
C6H14 |
2.18 |
|
C7H16 |
3.50 |
|
C8H18 |
6.39 |
|
C9H20 |
12.47 |
|
C10H22 |
25.22 |
- The Kovats retention index value for a compound can vary significantly from one column type to another; a selection of values for toluene can be found in the table below. Provide an explanation for why the Kovats retention index value varies between 1070 and 762?
- Why is there little to no variability in the Kovats retention index values for three of the five columns tabulated below?
Data obtained from:
http://www.pherobase.com/database/kovats/kovats-detail-toluene.php
|
Column |
Kovats # |
|---|---|
|
HP-101 |
762 |
|
HP-5MS |
762 |
|
HP-5 |
770 |
|
HP-20M |
1042 |
|
HP-FFAP |
1047 |
Contributors and Attributions
- Chris Harrison, San Diego State University (charrison@mail.sdsu.edu)
- Sourced from the Analytical Sciences Digital Library


