Biofuels Titration
- Page ID
- 282814
The growing area of renewable energy has now expanded into many markets. Biofuels are alternative fuels that are currently highly invested areas. Please watch the following Youtube video for more background on Biofuels:
For this activity, we will focus on Biodiesel. In the production process of biodiesel, the extracted fats /oils/in general grease (aka glycerides) from plants or algae are later chemically processed in a few different ways.
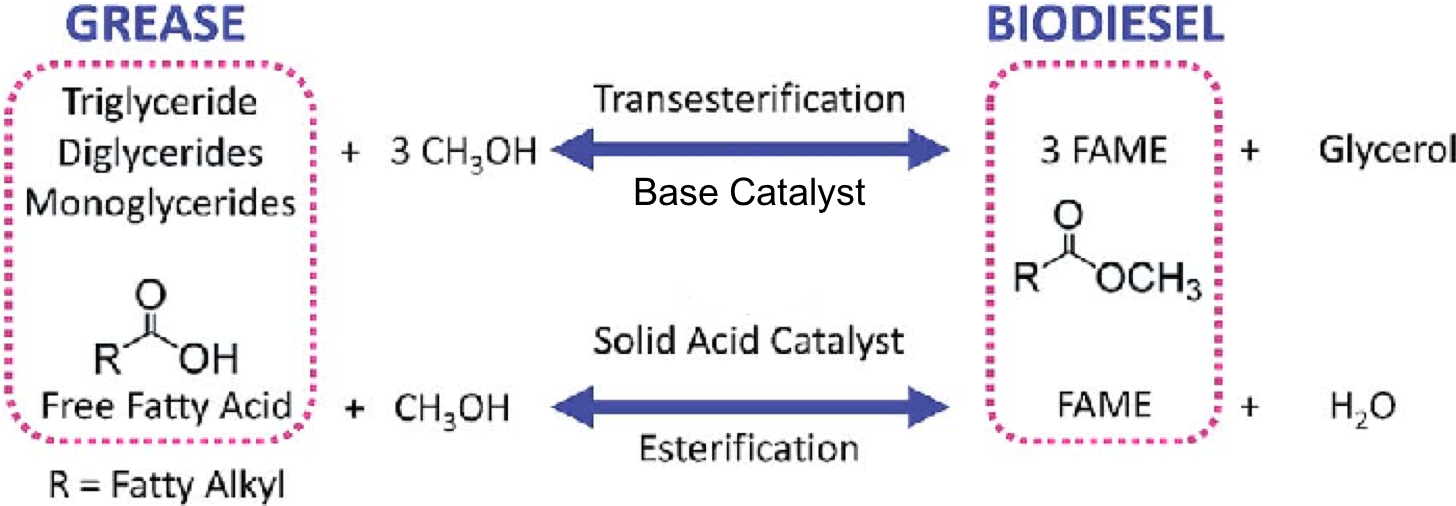
In the processing of the ‘grease’ there are two possible processes that can take place. If the majority of the ‘grease’ are forms of glycerides, Transesterification will take place. In this reaction, the glycerides are reacted with methanol (CH3OH) and a base catalyst (NaOH), resulting in a mixture of Fatty Acid Methyl Esters (FAME), the Biodiesel fuel, and Glycerol as a side product. If the majority of the ‘grease’ are Free Fatty acids, Esterification occurs. In Esterification, methanol with an Acid catalyst leads to FAME and water.
The selection of catalyst depends on the amount of Free Fatty Acids (FFA) originally present in the ‘grease’ mixture. The FFA content can be determined by using a simple titration method.
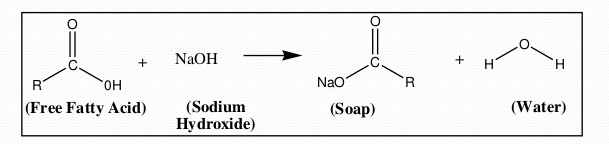
- FFA < 1% Base catalyst rxn is preformed
- FFA >1 % Acid Catalyst rxn is preformed
We can simplify our titration reaction as the following
\[\ce{HA + OH- → H2O + A-}\nonumber\]
Where HA is our weak acid (FFA) and OH- is our strong base.
For the purpose of our calculations we will pick Oleic acid as our FFA. Oleic acid (C18H34O2) has a pKa of 9.85, its molecular weight is 282.47 g/mol and has a density of 0.895g/mL. In our starting flask we add 800 mL of acid to 1L of water.
- Sketch the titration curve for our weak acid + strong base titration. Identify each point on the curve: initial pH, half-equivalence point, equivalence point, and after the equivalence point.
- Write the reaction of the Oleic acid before any addition of NaOH. Calculate the initial pH of Oleic acid.
- What is the volume of base that you need to reach the equivalence point if you are starting with 800 mL of acid and use 3M NaOH solution.
- Write the two reactions occurring at the Eq. point.
- Would you use the Ka or Kb in pH calculations at equivalence point in this case? Would you expect the pH to be acidic, basic, or neutral?
- Write the two reactions occurring at the Eq. point.
- Write the reaction occurring at half-equivalence point.
- What would be the pH at half-equivalence point.
- Now, calculate the pH of the solution after 300 mL and 900 mL of base addition.
- You are given a 4.5 L mixture of grease and wish to determine the best catalyst to convert the mixture into FAME. To assess the free fatty acid content (assume that all of the free fatty acid in the mixture is oleic acid (MW = 282.5 g/mol), you perform a titration with 3 M NaOH and find it requires 178 mL to reach the equivalence point. Determine the %FFA (by mass) in the mixture and decide which catalyst is most suitable. Assume that the grease mixture has a density of 0.925 g/mL.
Contributors and Attributions
- Asmira Alagic, Saint Louis University (admire.alagic@slu.edu)
- Sourced from the Analytical Sciences Digital Library


